Page 162 - Binder2
P. 162
Clinical Example: Celiac Disease Without
Immunosuppression
One of COUR’s lead programs targets celiac disease, a
condition where the immune system mounts an
inflammatory response to dietary gluten. This program
(TAK-101) is an excellent blueprint for exactly what has
been described in this chapter.
Rather than suppress the immune system or eliminate
gluten exposure entirely, COUR’s approach introduces
gliadin-derived peptides—the actual antigen driving the
immune response—via their nanoparticle system.
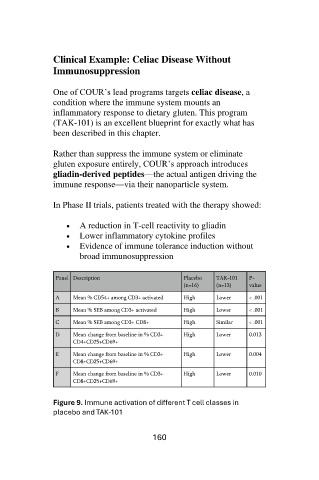
In Phase II trials, patients treated with the therapy showed:
• A reduction in T-cell reactivity to gliadin
• Lower inflammatory cytokine profiles
• Evidence of immune tolerance induction without
broad immunosuppression
Panel Description Placebo TAK-101 P-
(n=16) (n=13) value
A Mean % CD54+ among CD3+ activated High Lower < .001
B Mean % SEB among CD3+ activated High Lower < .001
C Mean % SEB among CD3+ CD8+ High Similar < .001
D Mean change from baseline in % CD3+ High Lower 0.013
CD4+CD25+CD69+
E Mean change from baseline in % CD3+ High Lower 0.004
CD8+CD25+CD69+
F Mean change from baseline in % CD3+ High Lower 0.010
CD8+CD25+CD69+
Figure 9. Immune activation of different T cell classes in
placebo and TAK-101
160