Page 4 - Vasoclick emagazine_Issue1_revised
P. 4
Improved Patient Reported Outcomes with Rivaroxaban in Cancer Associated Thrombosis 03
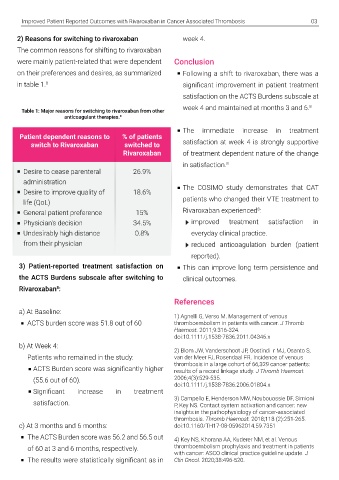
treatment option for patients with CAT, because follow-up. Anti-Clot Treatment Scale (ACTS) 2) Reasons for switching to rivaroxaban week 4.
9
it can be administered to patients as a fixed oral questionnaires were performed at baseline, week The common reasons for shifting to rivaroxaban
dose and without any requirement of routine 4, and months 3 and 6 for pairwise comparison were mainly patient-related that were dependent Conclusion
anticoagulation monitoring. 8 to mean scores at baseline (Figure 1). 8 on their preferences and desires, as summarized Following a shift to rivaroxaban, there was a
in table 1. 8 significant improvement in patient treatment
Cancer-associated thrOmboSIs – Study outcome: satisfaction on the ACTS Burdens subscale at
patient-reported outcoMes with rivarOx- Table 1: Major reasons for switching to rivaroxaban from other week 4 and maintained at months 3 and 6. 8
aban (COSIMO) study: 1) Percentage of patients who switched to anticoagulant therapies. 8
The COSIMO study was designed to evaluate Rivaroxaban from other therapies Patient dependent reasons to % of patients The immediate increase in treatment
patient satisfaction after planned change from Majority of patients changed to Rivaroxaban switch to Rivaroxaban switched to satisfaction at week 4 is strongly supportive
traditional anticoagulant therapy to rivaroxaban from LMWH therapy (96.65%), while few patients Rivaroxaban of treatment dependent nature of the change
therapy for cancer associated thrombosis changed from VKA and Fondaparinux as well Desire to cease parenteral 26.9% in satisfaction. 8
Factors underlying cancer associated treatment of CAT owing to superior efficacy and (CAT). 8, 9 (Figure 2). 8 administration The COSIMO study demonstrates that CAT
4, 5
thrombosis (CAT) safety. The major drawbacks associated with Desire to improve quality of 18.6%
VKA include strict requirement of monitoring of Study plan: life (QoL) patients who changed their VTE treatment to
Cancer promotes hypercoagulability in patients Rivaroxaban experienced :
8
international normalized ratio (INR) to track A prospective, non-interventional, single-arm General patient preference 15%
due to some or all of the following factors : anticoagulation status and interactions with food cohort study enrolled patients from 55 sites Physician’s decision 34.5% improved treatment satisfaction in
3
Long-term chemotherapy and drugs. 4,5 across Australia, Canada and Europe. 505 Undesirably high distance 0.8% everyday clinical practice.
8,9
Endothelial damage cancer patients who received rivaroxaban were from their physician reduced anticoagulation burden (patient
6
Obstruction to blood flow by tumor masses However, patients’ adherence towards LMWH included in the study. During analysis, ratings reported).
over oral anticoagulants is low thereby affecting were reverse coded; as a result, higher scores 3) Patient-reported treatment satisfaction on This can improve long term persistence and
Procoagulant microparticles released from the ACTS Burdens subscale after switching to
patient outcomes. Major reasons are as follows : reflected greater patient treatment satisfaction. clinical outcomes.
4
8
cancer cells Rivaroxaban :
8
Observations lasted for 6 months or until the
Comorbid conditions References
Inconvenient intravenous infusion requires participant withdrew consent, died, or was lost to
Advanced age recurrent hospital visits and clinical care a) At Baseline: 1) Agnelli G, Verso M. Management of venous
Restricted mobility High treatment cost ACTS burden score was 51.8 out of 60 thromboembolism in patients with cancer. J Thromb
Haemost. 2011;9:316-324.
doi:10.1111/j.1538-7836.2011.04346.x
Management of VTE in cancer patients Recently the international guidelines have been b) At Week 4: 2) Blom JW, Vanderschoot JP, Oostindi r MJ, Osanto S,
Due to high VTE recurrence risk in patients with updated to include recommendations for DOACs Patients who remained in the study: van der Meer FJ, Rosendaal FR. Incidence of venous
thrombosis in a large cohort of 66,329 cancer patients:
CAT, especially in the first 6 months, extended in patients with cancer and VTE. The American ACTS Burden score was significantly higher results of a record linkage study. J Thromb Haemost.
4,5
anticoagulation therapy has been recommended Society of hematology (ASH) 2021 guidelines (55.6 out of 60). 2006;4(3):529-535.
doi:10.1111/j.1538-7836.2006.01804.x
if the bleeding risk is low. 4,5 recommend the use of direct oral anticoagulants Significant increase in treatment 3) Campello E, Henderson MW, Noubouossie DF, Simioni
(DOACs) for the short term treatment of VTE in satisfaction. P, Key NS. Contact system activation and cancer: new
The previous guidelines included active cancer over low molecular weight heparin insights in the pathophysiology of cancer-associated
thrombosis. Thromb Haemost. 2018;118 (2):251-265.
low-molecular-weight heparin (LMWH) based (LMWH). 6 c) At 3 months and 6 months: doi:10.1160/TH17-08-05962014.59.7351
anticoagulation therapy over vitamin K The ACTS Burden score was 56.2 and 56.5 out 4) Key NS, Khorana AA, Kuderer NM, et al. Venous
antagonists (VKAs) for the initial and long-term Rivaroxaban is a promising and convenient of 60 at 3 and 6 months, respectively. thromboembolism prophylaxis and treatment in patients
with cancer: ASCO clinical practice guideline update. J
The results were statistically significant as in Clin Oncol. 2020;38:496-520.
5) National Comprehensive Cancer Network.
Cancer-associated venous thromboembolic disease,
Version 1.2020. National Comprehensive Cancer Network,
Inc.; 2020. Available at: https://www.nccn.org/profession
als/physi cian_gls/pdf/vte.pdf [accessed 22 March 2022].
6) Lyman GH, Carrier M, Ay C, Di Nisio M, Hicks LK,
Khorana AA, Leavitt AD, Lee AY, Macbeth F, Morgan RL,
Noble S. American Society of Hematology 2021
guidelines for management of venous thromboembolism:
prevention and treatment in patients with cancer. Blood
Adv. 2021; 5(4):927-974.
doi:10.1182/bloodadvances.2020003442
7) Yeh CH, Hogg K, Weitz JI. Overview of the new oral
anticoagulants: opportunities and challenges. Arterioscler
Thromb Vasc Biol. 2015;35:1056-1065.
8) Cohen AT, Maraveyas A, Beyer-Westendorf J, Lee AY,
Folkerts K, Abdelgawwad K, De Sanctis Y, Fatoba S,
Bamber L, Bach M, Mantovani LG. Patient-reported
outcomes associated with changing to rivaroxaban for
the treatment of cancer-associated venous
thromboembolism–The COSIMO study. Thromb Res.
2021; 206; 1-4. doi: 10.1016/j.thromres.2021.06.021
9) Maraveyas A, Beyer-Westendorf J, Lee AY, et al.
Cancer-Associated ThrOmboSIs - Patient-Reported
OutcoMes With RivarOxaban (COSIMO) - Baseline
characteristics and clinical outcomes. Res Pract Thromb
Haemost. 2021;5(8):e12604. doi:10.1002/rth2.12604