Page 7 - Vasoclick emagazine_Issue1_revised
P. 7
2) Reasons for switching to rivaroxaban
week 4.
follow-up. Anti-Clot Treatment Scale (ACTS)
treatment option for patients with CAT, because
9
The common reasons for shifting to rivaroxaban
it can be administered to patients as a fixed oral
questionnaires were performed at baseline, week
were mainly patient-related that were dependent
dose and without any requirement of routine
4, and months 3 and 6 for pairwise comparison
on their preferences and desires, as summarized
to mean scores at baseline (Figure 1).
Following a shift to rivaroxaban, there was a
anticoagulation monitoring.
8
8
in table 1.
significant improvement in patient treatment
8
Study outcome:
Cancer-associated thrOmboSIs –
satisfaction on the ACTS Burdens subscale at
patient-reported outcoMes with rivarOx-
week 4 and maintained at months 3 and 6.
aban (COSIMO) study:
1) Percentage of patients who switched to
The immediate increase in treatment
Rivaroxaban from other therapies
The COSIMO study was designed to evaluate
satisfaction at week 4 is strongly supportive
Majority of patients changed to Rivaroxaban
patient satisfaction after planned change from
of treatment dependent nature of the change
from LMWH therapy (96.65%), while few patients
traditional anticoagulant therapy to rivaroxaban
in satisfaction.
changed from VKA and Fondaparinux as well
therapy for cancer associated thrombosis
(Figure 2).
8
(CAT).
8, 9
treatment of CAT owing to superior efficacy and
Factors underlying cancer associated
The COSIMO study demonstrates that CAT
safety. The major drawbacks associated with
4, 5
thrombosis (CAT)
patients who changed their VTE treatment to
VKA include strict requirement of monitoring of
Study plan:
Cancer promotes hypercoagulability in patients
Rivaroxaban experienced :
8
international normalized ratio (INR) to track
A prospective, non-interventional, single-arm
in
improved
due to some or all of the following factors :
3
anticoagulation status and interactions with food
cohort study enrolled patients from 55 sites
everyday clinical practice.
Long-term chemotherapy
and drugs.
4,5
across Australia, Canada and Europe. 505
8,9
reduced anticoagulation burden (patient
Endothelial damage
cancer patients who received rivaroxaban were
reported).
However, patients’ adherence towards LMWH
included in the study. During analysis, ratings
6
Obstruction to blood flow by tumor masses
3) Patient-reported treatment satisfaction on
This can improve long term persistence and
over oral anticoagulants is low thereby affecting
were reverse coded; as a result, higher scores
Procoagulant microparticles released from
the ACTS Burdens subscale after switching to
clinical outcomes.
patient outcomes. Major reasons are as follows :
4
reflected greater patient treatment satisfaction.
8
cancer cells
Rivaroxaban :
8
Observations lasted for 6 months or until the
Comorbid conditions
References
Inconvenient intravenous infusion requires
participant withdrew consent, died, or was lost to
a) At Baseline:
Advanced age
recurrent hospital visits and clinical care
1) Agnelli G, Verso M. Management of venous
ACTS burden score was 51.8 out of 60
thromboembolism in patients with cancer. J Thromb
Restricted mobility
High treatment cost
Haemost. 2011;9:316-324.
doi:10.1111/j.1538-7836.2011.04346.x
b) At Week 4:
Management of VTE in cancer patients
Recently the international guidelines have been
2) Blom JW, Vanderschoot JP, Oostindi r MJ, Osanto S,
Patients who remained in the study:
van der Meer FJ, Rosendaal FR. Incidence of venous
updated to include recommendations for DOACs
Due to high VTE recurrence risk in patients with
thrombosis in a large cohort of 66,329 cancer patients:
ACTS Burden score was significantly higher
results of a record linkage study. J Thromb Haemost.
CAT, especially in the first 6 months, extended
in patients with cancer and VTE. The American
4,5
2006;4(3):529-535.
(55.6 out of 60).
anticoagulation therapy has been recommended
Society of hematology (ASH) 2021 guidelines
doi:10.1111/j.1538-7836.2006.01804.x
Significant
recommend the use of direct oral anticoagulants
if the bleeding risk is low.
4,5
3) Campello E, Henderson MW, Noubouossie DF, Simioni
satisfaction.
P, Key NS. Contact system activation and cancer: new
(DOACs) for the short term treatment of VTE in
insights in the pathophysiology of cancer-associated
active cancer over low molecular weight heparin
guidelines
The
included
previous
thrombosis. Thromb Haemost. 2018;118 (2):251-265.
c) At 3 months and 6 months:
doi:10.1160/TH17-08-05962014.59.7351
low-molecular-weight heparin (LMWH) based
(LMWH).
6
The ACTS Burden score was 56.2 and 56.5 out
4) Key NS, Khorana AA, Kuderer NM, et al. Venous
anticoagulation
K
vitamin
over
therapy
thromboembolism prophylaxis and treatment in patients
of 60 at 3 and 6 months, respectively.
antagonists (VKAs) for the initial and long-term
Rivaroxaban is a promising and convenient
with cancer: ASCO clinical practice guideline update. J
The results were statistically significant as in
Clin Oncol. 2020;38:496-520.
Vasoclick, Edition 1 increase in treatment Conclusion 8 treatment satisfaction 8 06
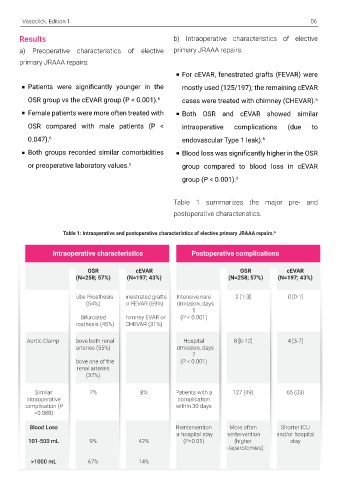
5) National Comprehensive Cancer Network. Results b) Intraoperative characteristics of elective
Cancer-associated venous thromboembolic disease, a) Preoperative characteristics of elective primary JRAAA repairs:
Version 1.2020. National Comprehensive Cancer Network,
Inc.; 2020. Available at: https://www.nccn.org/profession primary JRAAA repairs:
als/physi cian_gls/pdf/vte.pdf [accessed 22 March 2022].
For cEVAR, fenestrated grafts (FEVAR) were
6) Lyman GH, Carrier M, Ay C, Di Nisio M, Hicks LK,
Khorana AA, Leavitt AD, Lee AY, Macbeth F, Morgan RL, Patients were significantly younger in the mostly used (125/197); the remaining cEVAR
Noble S. American Society of Hematology 2021
guidelines for management of venous thromboembolism: OSR group vs the cEVAR group (P < 0.001). 6 cases were treated with chimney (CHEVAR). 6
prevention and treatment in patients with cancer. Blood
Adv. 2021; 5(4):927-974. Female patients were more often treated with Both OSR and cEVAR showed similar
doi:10.1182/bloodadvances.2020003442
OSR compared with male patients (P < intraoperative complications (due to
7) Yeh CH, Hogg K, Weitz JI. Overview of the new oral 0.047). 6 endovascular Type 1 leak). 6
anticoagulants: opportunities and challenges. Arterioscler
Thromb Vasc Biol. 2015;35:1056-1065. Both groups recorded similar comorbidities Blood loss was significantly higher in the OSR
8) Cohen AT, Maraveyas A, Beyer-Westendorf J, Lee AY, or preoperative laboratory values. group compared to blood loss in cEVAR
6
Folkerts K, Abdelgawwad K, De Sanctis Y, Fatoba S,
Bamber L, Bach M, Mantovani LG. Patient-reported group (P < 0.001). 6
outcomes associated with changing to rivaroxaban for
the treatment of cancer-associated venous
thromboembolism–The COSIMO study. Thromb Res.
2021; 206; 1-4. doi: 10.1016/j.thromres.2021.06.021 Table 1 summarizes the major pre- and
postoperative characteristics.
9) Maraveyas A, Beyer-Westendorf J, Lee AY, et al.
Cancer-Associated ThrOmboSIs - Patient-Reported
OutcoMes With RivarOxaban (COSIMO) - Baseline Table 1: Intraoperative and postoperative characteristics of elective primary JRAAA repairs. 6
characteristics and clinical outcomes. Res Pract Thromb
Haemost. 2021;5(8):e12604. doi:10.1002/rth2.12604
Intraoperative characteristics Postoperative complications
OSR cEVAR OSR cEVAR
(N=258; 57%) (N=197; 43%) (N=258; 57%) (N=197; 43%)
Tube Prosthesis Fenestrated grafts Intensive care 2 [1-3] 0 [0-1]
(54%) or FEVAR (69%) admission, days
1
Bifurcated Chimney EVAR or (P < 0.001)
Prosthesis (45%) CHEVAR (31%)
Aortic Clamp: Above both renal Hospital 8 [6-12] 4 [3-7]
arteries (55%) admission, days
7
Above one of the (P < 0.001)
renal arteries
(37%)
Similar 7% 8% Patients with a 127 (49) 65 (33)
intraoperative complication
complication (P within 30 days
=0.088)
Blood Loss Reintervention More often Shorter ICU
or hospital stay reintervention and/or hospital
101-500 mL 9% 42% (P<0.01) (higher stay
re-laparotomies)
>1000 mL 67% 14%