Page 54 - E-LKM ACID BASE
P. 54
Lembar Kerja Mahasiswa - Asam Basa
E
U
L
A
V
A
N
EVALUATION
O
T
I
1. Does the reaction between acid and base always produce
a product in the form of salts? Explain your answer based
on the theories of Arrhenius, Bronsted-Lowry, and Lewis!
2. When making food, we often use baking soda (NaHCO ) as
3
a developer ingredient, especially cake developers. Why
can NaHCO be used as a food developer and if there is no
3
NaHCO is there another alternative as a food developer?
3
Explain your answer!
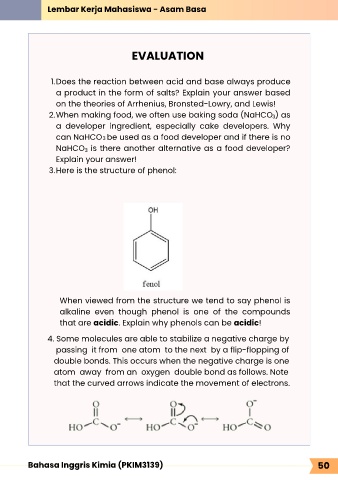
3. Here is the structure of phenol:
When viewed from the structure we tend to say phenol is
alkaline even though phenol is one of the compounds
that are acidic. Explain why phenols can be acidic!
4. Some molecules are able to stabilize a negative charge by
passing it from one atom to the next by a flip-flopping of
double bonds. This occurs when the negative charge is one
atom away from an oxygen double bond as follows. Note
that the curved arrows indicate the movement of electrons.
Bahasa Inggris Kimia (PKIM3139) 50