Page 4 - Understanding light, colour and hair colour
P. 4
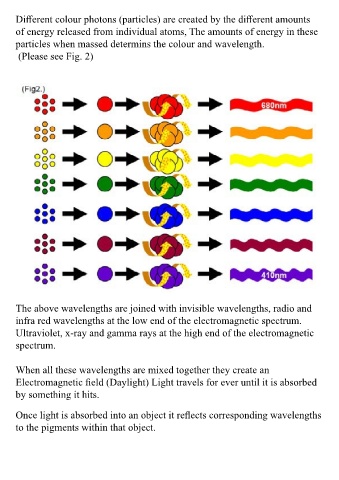
Different colour photons (particles) are created by the different amounts All wavelengths are registered on a spectrum called the Electromagnetic
of energy released from individual atoms, The amounts of energy in these Spectrum.(Please see Fig. 3)
particles when massed determins the colour and wavelength.
(Please see Fig. 2)
The above wavelengths are joined with invisible wavelengths, radio and
infra red wavelengths at the low end of the electromagnetic spectrum.
Ultraviolet, x-ray and gamma rays at the high end of the electromagnetic You will notice from this linear version of the electromagnetic spectrum,
spectrum. it actually contains a visible spectrum and an invisible spectrum.
The visible spectrum contains wavelengths from 400nm to 700nm. The
When all these wavelengths are mixed together they create an
Electromagnetic field (Daylight) Light travels for ever until it is absorbed invisible spectrum contains infra red and radio wavelengths between
by something it hits. 700nm to 1metre or greater at the low end of the electromagnetic
Spectrum, with ultra violet rays, x-rays and gamma rays from 400nm to
Once light is absorbed into an object it reflects corresponding wavelengths 100th of a nanometer at the high end of the electromagnetic spectrum.
to the pigments within that object.