Page 27 - YORAM RUDY BOOK FINAL
P. 27
P. 27
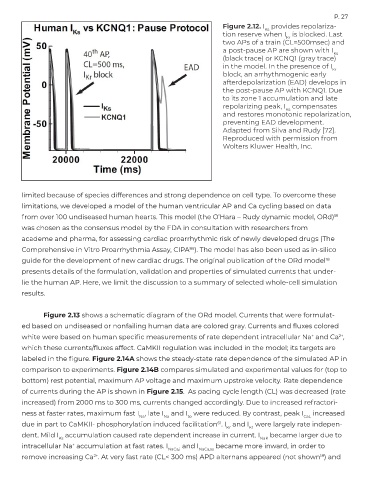
Figure 2.12. I provides repolariza-
Ks
tion reserve when I is blocked. Last
Kr
two APs of a train (CL=500msec) and
a post-pause AP are shown with I
Ks
(black trace) or KCNQ1 (gray trace)
in the model. In the presence of I
Kr
block, an arrhythmogenic early
afterdepolarization (EAD) develops in
the post-pause AP with KCNQ1. Due
to its zone 1 accumulation and late
repolarizing peak, I compensates
Ks
and restores monotonic repolarization,
preventing EAD development.
Adapted from Silva and Rudy [72].
Reproduced with permission from
Wolters Kluwer Health, Inc.
limited because of species differences and strong dependence on cell type. To overcome these
limitations, we developed a model of the human ventricular AP and Ca cycling based on data
from over 100 undiseased human hearts. This model (the O’Hara – Rudy dynamic model, ORd)
18
was chosen as the consensus model by the FDA in consultation with researchers from
academe and pharma, for assessing cardiac proarrhythmic risk of newly developed drugs (The
Comprehensive in Vitro Proarrhythmia Assay, CIPA ). The model has also been used as in-silico
86
guide for the development of new cardiac drugs. The original publication of the ORd model
18
presents details of the formulation, validation and properties of simulated currents that under-
lie the human AP. Here, we limit the discussion to a summary of selected whole-cell simulation
results.
Figure 2.13 shows a schematic diagram of the ORd model. Currents that were formulat-
ed based on undiseased or nonfailing human data are colored gray. Currents and fluxes colored
white were based on human specific measurements of rate dependent intracellular Na and Ca ,
2+
+
which these currents/fluxes affect. CaMKII regulation was included in the model; its targets are
labeled in the figure. Figure 2.14A shows the steady-state rate dependence of the simulated AP in
comparison to experiments. Figure 2.14B compares simulated and experimental values for (top to
bottom) rest potential, maximum AP voltage and maximum upstroke velocity. Rate dependence
of currents during the AP is shown in Figure 2.15. As pacing cycle length (CL) was decreased (rate
increased) from 2000 ms to 300 ms, currents changed accordingly. Due to increased refractori-
ness at faster rates, maximum fast I , late I and I were reduced. By contrast, peak I CaL increased
Na
Na
to
due in part to CaMKII- phosphorylation induced facilitation . I and I were largely rate indepen-
61
K1
Kr
dent. Mild I accumulation caused rate dependent increase in current. I NaK became larger due to
Ks
intracellular Na accumulation at fast rates. I NaCa,i and I NaCa,ss became more inward, in order to
+
remove increasing Ca . At very fast rate (CL< 300 ms) APD alternans appeared (not shown ) and
2+
18