Page 41 - YORAM RUDY BOOK FINAL
P. 41
P. 41
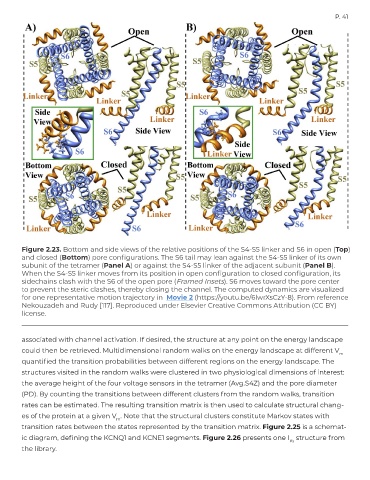
Figure 2.23. Bottom and side views of the relative positions of the S4-S5 linker and S6 in open (Top)
and closed (Bottom) pore configurations. The S6 tail may lean against the S4-S5 linker of its own
subunit of the tetramer (Panel A) or against the S4-S5 linker of the adjacent subunit (Panel B).
When the S4-S5 linker moves from its position in open configuration to closed configuration, its
sidechains clash with the S6 of the open pore (Framed Insets). S6 moves toward the pore center
to prevent the steric clashes, thereby closing the channel. The computed dynamics are visualized
for one representative motion trajectory in Movie 2 (https://youtu.be/6lwrXsCzY-8). From reference
Nekouzadeh and Rudy [117]. Reproduced under Elsevier Creative Commons Attribution (CC BY)
license.
associated with channel activation. If desired, the structure at any point on the energy landscape
could then be retrieved. Multidimensional random walks on the energy landscape at different V
m
quantified the transition probabilities between different regions on the energy landscape. The
structures visited in the random walks were clustered in two physiological dimensions of interest:
the average height of the four voltage sensors in the tetramer (Avg.S4Z) and the pore diameter
(PD). By counting the transitions between different clusters from the random walks, transition
rates can be estimated. The resulting transition matrix is then used to calculate structural chang-
es of the protein at a given V . Note that the structural clusters constitute Markov states with
m
transition rates between the states represented by the transition matrix. Figure 2.25 is a schemat-
ic diagram, defining the KCNQ1 and KCNE1 segments. Figure 2.26 presents one I structure from
Ks
the library.