Page 64 - YORAM RUDY BOOK FINAL
P. 64
P. 64
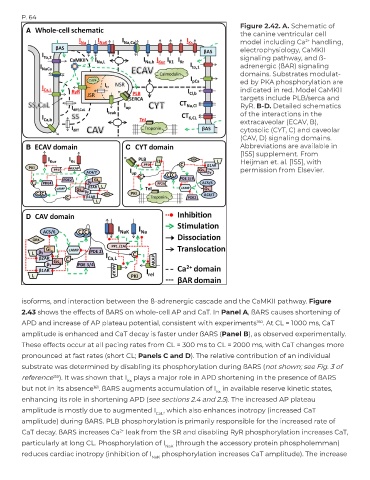
Figure 2.42. A. Schematic of
the canine ventricular cell
model including Ca handling,
2+
electrophysiology, CaMKII
signaling pathway, and ß-
adrenergic (ßAR) signaling
domains. Substrates modulat-
ed by PKA phosphorylation are
indicated in red. Model CaMKII
targets include PLB/serca and
RyR. B-D. Detailed schematics
of the interactions in the
extracaveolar (ECAV, B),
cytosolic (CYT, C) and caveolar
(CAV, D) signaling domains.
Abbreviations are available in
[155] supplement. From
Heijman et. al. [155], with
permission from Elsevier.
isoforms, and interaction between the ß-adrenergic cascade and the CaMKII pathway. Figure
2.43 shows the effects of ßARS on whole-cell AP and CaT. In Panel A, ßARS causes shortening of
APD and increase of AP plateau potential, consistent with experiments . At CL = 1000 ms, CaT
160
amplitude is enhanced and CaT decay is faster under ßARS (Panel B), as observed experimentally.
These effects occur at all pacing rates from CL = 300 ms to CL = 2000 ms, with CaT changes more
pronounced at fast rates (short CL; Panels C and D). The relative contribution of an individual
substrate was determined by disabling its phosphorylation during ßARS (not shown; see Fig. 3 of
reference ). It was shown that I plays a major role in APD shortening in the presence of ßARS
155
Ks
but not in its absence . ßARS augments accumulation of I in available reserve kinetic states,
161
Ks
enhancing its role in shortening APD (see sections 2.4 and 2.5). The increased AP plateau
amplitude is mostly due to augmented I CaL , which also enhances inotropy (increased CaT
amplitude) during ßARS. PLB phosphorylation is primarily responsible for the increased rate of
CaT decay. ßARS increases Ca leak from the SR and disabling RyR phosphorylation increases CaT,
2+
particularly at long CL. Phosphorylation of I NaK (through the accessory protein phospholemman)
reduces cardiac inotropy (inhibition of I NaK phosphorylation increases CaT amplitude). The increase