Page 15 - Fluid, Electrolyte, and Acid-Base Disorders in Small Animal Practice
P. 15
Applied Physiology of Body Fluids in Dogs and Cats 5
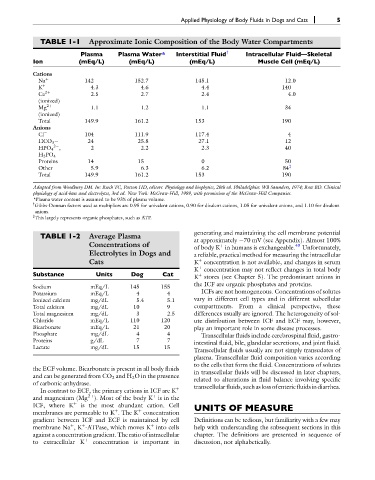
TABLE 1-1 Approximate Ionic Composition of the Body Water Compartments
Plasma Plasma Water* Interstitial Fluid { Intracellular Fluid—Skeletal
Ion (mEq/L) (mEq/L) (mEq/L) Muscle Cell (mEq/L)
Cations
Na þ 142 152.7 145.1 12.0
K þ 4.3 4.6 4.4 140
Ca 2þ 2.5 2.7 2.4 4.0
(ionized)
Mg 2þ 1.1 1.2 1.1 34
(ionized)
Total 149.9 161.2 153 190
Anions
Cl 104 111.9 117.4 4
HCO 3 24 25.8 27.1 12
2
HPO 4 , 2 2.2 2.3 40
H 2 PO 4
Proteins 14 15 0 50
{
Other 5.9 6.3 6.2 84
Total 149.9 161.2 153 190
Adapted from Woodbury DM. In: Ruch TC, Patton HD, editors. Physiology and biophysics, 20th ed. Philadelphia: WB Saunders, 1974; Rose BD. Clinical
physiology of acid-base and electrolytes, 3rd ed. New York: McGraw-Hill, 1989, with permission of the McGraw-Hill Companies.
*Plasma water content is assumed to be 93% of plasma volume.
{
Gibbs-Donnan factors used as multipliers are 0.95 for univalent cations, 0.90 for divalent cations, 1.05 for univalent anions, and 1.10 for divalent
anions.
{
This largely represents organic phosphates, such as ATP.
TABLE 1-2 Average Plasma generating and maintaining the cell membrane potential
at approximately 70 mV (see Appendix). Almost 100%
Concentrations of 48
of body K in humans is exchangeable. Unfortunately,
þ
Electrolytes in Dogs and a reliable, practical method for measuring the intracellular
Cats K concentration is not available, and changes in serum
þ
þ
K concentration may not reflect changes in total body
Substance Units Dog Cat þ
K stores (see Chapter 5). The predominant anions in
the ICF are organic phosphates and proteins.
Sodium mEq/L 145 155
Potassium mEq/L 4 4 ICFs are not homogeneous. Concentrations of solutes
Ionized calcium mg/dL 5.4 5.1 vary in different cell types and in different subcellular
Total calcium mg/dL 10 9 compartments. From a clinical perspective, these
Total magnesium mg/dL 3 2.5 differences usually are ignored. The heterogeneity of sol-
Chloride mEq/L 110 120 ute distribution between ICF and ECF may, however,
Bicarbonate mEq/L 21 20 play an important role in some disease processes.
Phosphate mg/dL 4 4 Transcellular fluids include cerebrospinal fluid, gastro-
Proteins g/dL 7 7
intestinal fluid, bile, glandular secretions, and joint fluid.
Lactate mg/dL 15 15
Transcellular fluids usually are not simply transudates of
plasma. Transcellular fluid composition varies according
to the cells that form the fluid. Concentrations of solutes
the ECF volume. Bicarbonate is present in all body fluids in transcellular fluids will be discussed in later chapters,
and can be generated from CO 2 and H 2 O in the presence related to alterations in fluid balance involving specific
of carbonic anhydrase.
transcellular fluids, such asloss ofentericfluids indiarrhea.
In contrast to ECF, the primary cations in ICF are K þ
and magnesium (Mg 2þ ). Most of the body K is in the
þ
ICF, where K þ is the most abundant cation. Cell UNITS OF MEASURE
membranes are permeable to K . The K concentration
þ
þ
gradient between ICF and ECF is maintained by cell Definitions can be tedious, but familiarity with a few may
þ
membrane Na ,K -ATPase, which moves K into cells help with understanding the subsequent sections in this
þ
þ
against a concentration gradient. The ratio of intracellular chapter. The definitions are presented in sequence of
to extracellular K þ concentration is important in discussion, not alphabetically.