Page 439 - The Toxicology of Fishes
P. 439
Toxic Responses of the Fish Nervous System 419
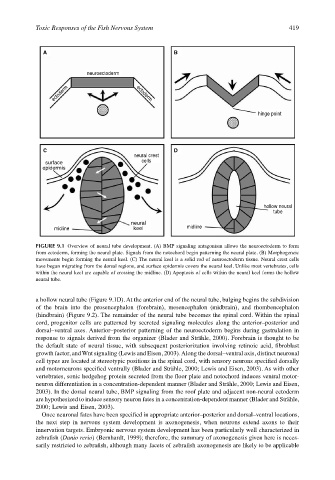
A B
neuroectoderm
ectoderm ectoderm
hinge point
C D
neural crest
surface cells
epidermis
hollow neural
tube
neural
midline keel midline
FIGURE 9.1 Overview of neural tube development. (A) BMP signaling antagonism allows the neuroectoderm to form
from ectoderm, forming the neural plate. Signals from the notochord begin patterning the neural plate. (B) Morphogeneic
movements begin forming the neural keel. (C) The neural keel is a solid rod of neuroectoderm tissue. Neural crest cells
have begun migrating from the dorsal regions, and surface epidermis covers the neural keel. Unlike most vertebrates, cells
within the neural keel are capable of crossing the midline. (D) Apoptosis of cells within the neural keel forms the hollow
neural tube.
a hollow neural tube (Figure 9.1D). At the anterior end of the neural tube, bulging begins the subdivision
of the brain into the prosencephalon (forebrain), mesencephalon (midbrain), and rhombencephalon
(hindbrain) (Figure 9.2). The remainder of the neural tube becomes the spinal cord. Within the spinal
cord, progenitor cells are patterned by secreted signaling molecules along the anterior–posterior and
dorsal–ventral axes. Anterior–posterior patterning of the neuroectoderm begins during gastrulation in
response to signals derived from the organizer (Blader and Strähle, 2000). Forebrain is thought to be
the default state of neural tissue, with subsequent posteriorization involving retinoic acid, fibroblast
growth factor, and Wnt signaling (Lewis and Eisen, 2003). Along the dorsal–ventral axis, distinct neuronal
cell types are located at stereotypic positions in the spinal cord, with sensory neurons specified dorsally
and motorneurons specified ventrally (Blader and Strähle, 2000; Lewis and Eisen, 2003). As with other
vertebrates, sonic hedgehog protein secreted from the floor plate and notochord induces ventral motor-
neuron differentiation in a concentration-dependent manner (Blader and Strähle, 2000; Lewis and Eisen,
2003). In the dorsal neural tube, BMP signaling from the roof plate and adjacent non-neural ectoderm
are hypothesized to induce sensory neuron fates in a concentration-dependent manner (Blader and Strähle,
2000; Lewis and Eisen, 2003).
Once neuronal fates have been specified in appropriate anterior–posterior and dorsal–ventral locations,
the next step in nervous system development is axonogenesis, when neurons extend axons to their
innervation targets. Embryonic nervous system development has been particularly well characterized in
zebrafish (Danio rerio) (Bernhardt, 1999); therefore, the summary of axonogenesis given here is neces-
sarily restricted to zebrafish, although many facets of zebrafish axonogenesis are likely to be applicable