Page 82 - The Toxicology of Fishes
P. 82
62 The Toxicology of Fishes
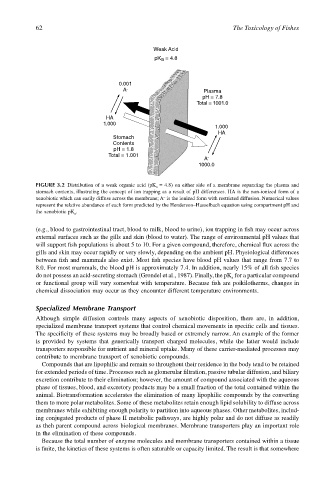
Weak Acid
pK a = 4.8
0.001
A – Plasma
pH = 7.8
Total = 1001.0
HA
1.000
1.000
HA
Stomach
Contents
pH = 1.8
Total = 1.001
A –
1000.0
FIGURE 3.2 Distribution of a weak organic acid (pK a = 4.8) on either side of a membrane separating the plasma and
stomach contents, illustrating the concept of ion trapping as a result of pH differences. HA is the non-ionized form of a
xenobiotic which can easily diffuse across the membrane; A is the ionized form with restricted diffusion. Numerical values
–
represent the relative abundance of each form predicted by the Henderson–Hasselbach equation using compartment pH and
the xenobiotic pK a .
(e.g., blood to gastrointestinal tract, blood to milk, blood to urine), ion trapping in fish may occur across
external surfaces such as the gills and skin (blood to water). The range of environmental pH values that
will support fish populations is about 5 to 10. For a given compound, therefore, chemical flux across the
gills and skin may occur rapidly or very slowly, depending on the ambient pH. Physiological differences
between fish and mammals also exist. Most fish species have blood pH values that range from 7.7 to
8.0. For most mammals, the blood pH is approximately 7.4. In addition, nearly 15% of all fish species
do not possess an acid-secreting stomach (Grondel et al., 1987). Finally, the pK for a particular compound
a
or functional group will vary somewhat with temperature. Because fish are poikilotherms, changes in
chemical dissociation may occur as they encounter different temperature environments.
Specialized Membrane Transport
Although simple diffusion controls many aspects of xenobiotic disposition, there are, in addition,
specialized membrane transport systems that control chemical movements in specific cells and tissues.
The specificity of these systems may be broadly based or extremely narrow. An example of the former
is provided by systems that generically transport charged molecules, while the latter would include
transporters responsible for nutrient and mineral uptake. Many of these carrier-mediated processes may
contribute to membrane transport of xenobiotic compounds.
Compounds that are lipophilic and remain so throughout their residence in the body tend to be retained
for extended periods of time. Processes such as glomerular filtration, passive tubular diffusion, and biliary
excretion contribute to their elimination; however, the amount of compound associated with the aqueous
phase of tissues, blood, and excretory products may be a small fraction of the total contained within the
animal. Biotransformation accelerates the elimination of many lipophilic compounds by the converting
them to more polar metabolites. Some of these metabolites retain enough lipid solubility to diffuse across
membranes while exhibiting enough polarity to partition into aqueous phases. Other metabolites, includ-
ing conjugated products of phase II metabolic pathways, are highly polar and do not diffuse as readily
as theh parent compound across biological membranes. Membrane transporters play an important role
in the elimination of these compounds.
Because the total number of enzyme molecules and membrane transporters contained within a tissue
is finite, the kinetics of these systems is often saturable or capacity limited. The result is that somewhere