Page 1145 - Veterinary Toxicology, Basic and Clinical Principles, 3rd Edition
P. 1145
Ionophores Chapter | 78 1077
VetBooks.ir The pattern of metabolites in cattle and rats is similar. and will be due primarily to the parent compound.
Furthermore, when residues, primarily the parent com-
Based on radiolabeled studies in steers and dairy cows,
the liver had the highest total monensin residue following
pound, are present in animal products intended for human
zero withdrawal (Davison, 1984; Donoho, 1984; consumption, they will be at minimal levels for which a
Kennington et al., 1995). Parent monensin and five meta- sufficient margin of safety has been demonstrated in the
bolites were identified in liver, bile and/or feces from toxicology studies (Novilla, 2004).
monensin-treated steers and dairy cows. Studies have Pharmacokinetic studies with other marketed ionophores
shown that metabolite M-1 (o-desmethyl monensin) was generated data similar to that of monensin. Results of these
20 times less active than parent monensin, the marker res- studies in the FOI summaries facilitated determination of the
idue. Hence, the suspected first step in monensin metabo- safe residue concentration, acceptable daily intake, marker
lism appears to eliminate most of the biological activity. residue and tolerance, and withdrawal time by the US FDA.
Plasma levels of monensin are low and decline rapidly
in cattle treated with monensin. In cattle administered
14
C-monensin, residues of radioactivity depleted rapidly MECHANISM OF ACTION
from the tissues. In a recent tissue residue study, no mon-
1
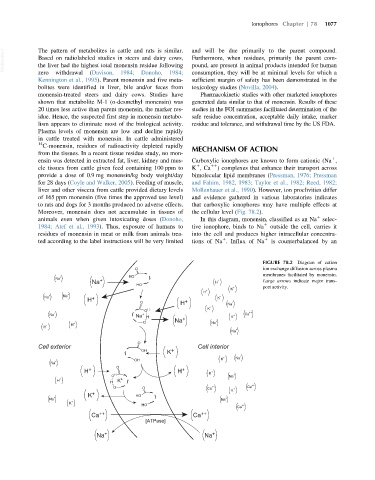
ensin was detected in extracted fat, liver, kidney and mus- Carboxylic ionophores are known to form cationic (Na ,
1
cle tissues from cattle given feed containing 100 ppm to K ,Ca 11 ) complexes that enhance their transport across
provide a dose of 0.9 mg monensin/kg body weight/day bimolecular lipid membranes (Pressman, 1976; Pressman
for 28 days (Coyle and Walker, 2005). Feeding of muscle, and Fahim, 1982, 1983; Taylor et al., 1982; Reed, 1982;
liver and other viscera from cattle provided dietary levels Mollenhauer et al., 1990). However, ion proclivities differ
of 165 ppm monensin (five times the approved use level) and evidence gathered in various laboratories indicates
to rats and dogs for 3 months produced no adverse effects. that carboxylic ionophores may have multiple effects at
Moreover, monensin does not accumulate in tissues of the cellular level (Fig. 78.2).
1
animals even when given intoxicating doses (Donoho, In this diagram, monensin, classified as an Na selec-
1
1984; Atef et al., 1993). Thus, exposure of humans to tive ionophore, binds to Na outside the cell, carries it
residues of monensin in meat or milk from animals trea- into the cell and produces higher intracellular concentra-
1 1
ted according to the label instructions will be very limited tions of Na . Influx of Na is counterbalanced by an
FIGURE 78.2 Diagram of cation
O ion exchange diffusion across plasma
membranes facilitated by monensin.
+ HO
Na +
Na H + Large arrows indicate major trans-
HO port activity.
K +
H +
Na + Na + + K +
H
O H + Na +
(–) K +
O
+ ++
Na Na + H K + Ca
O Na + Na +
+ H +
H
+
Na
Cell exterior O Cell interior
OH K +
+
+
Na
OH K
+
Na
O
H + H + K +
O (–) Na +
H + H K +
++
O O Ca ++ K + Ca
K + HO
+
Na Na +
K + HO
Ca ++
Ca ++ Ca ++
[ATPase]
Na + Na +