Page 1149 - Veterinary Toxicology, Basic and Clinical Principles, 3rd Edition
P. 1149
Ionophores Chapter | 78 1081
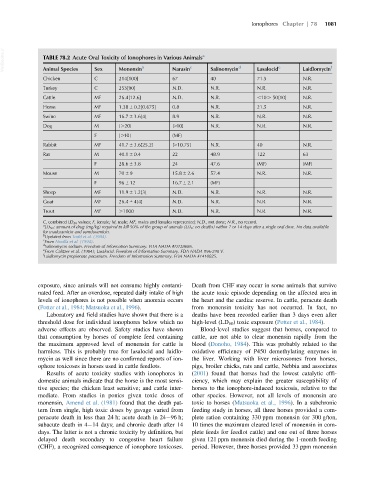
VetBooks.ir TABLE 78.2 Acute Oral Toxicity of Ionophores in Various Animals a Salinomycin d Lasalocid e Laidlomycin f
b
c
Sex
Animal Species
Monensin
Narasin
Chicken C 214[100] 67 40 71.5 N.R.
Turkey C 253[90] N.D. N.R. N.R. N.R.
Cattle MF 26.4[12.6] N.D. N.R. ,10. 50[10] N.R.
Horse MF 1.38 6 0.2[0.675] 0.8 N.R. 21.5 N.R.
Swine MF 16.7 6 3.6[4] 8.9 N.R. N.R. N.R.
Dog M [.20] [.10] N.R. N.R. N.R.
F [.10] (MF)
Rabbit MF 41.7 6 3.6[25.2] [.10.75] N.R. 40 N.R.
Rat M 40.1 6 0.4 22 48.9 122 63
F 28.6 6 3.8 24 47.6 (MF) (MF)
Mouse M 70 6 9 15.8 6 2.6 57.4 N.R. N.R.
F 96 6 12 16.7 6 2.1 (MF)
Sheep MF 11.9 6 1.2[3] N.D. N.R. N.R. N.R.
Goat MF 26.4 6 4[4] N.D. N.R. N.R. N.R.
Trout MF .1000 N.D. N.R. N.R. N.R.
C, combined LD 50 values; F, female; M, male; MF, males and females represented; N.D., not done; N.R., no record.
a
LD 50 : amount of drug (mg/kg) required to kill 50% of the group of animals (LD 0 : no deaths) within 7 or 14 days after a single oral dose. No data available
for maduramicin and semduramicin.
b
Updated from Todd et al. (1984).
c
From Novilla et al. (1994).
d
Salinomycin sodium. Freedom of Information Summary. FDA NADA #D128686.
e
From Galitzer et al. (1984); Lasalocid. Freedom of Information Summary. FDA NADA #96-298 V.
f
Laidlomycin propionate potassium. Freedom of Information Summary. FDA NADA #1410025.
exposure, since animals will not consume highly contami- Death from CHF may occur in some animals that survive
nated feed. After an overdose, repeated daily intake of high the acute toxic episode depending on the affected area in
levels of ionophores is not possible when anorexia occurs the heart and the cardiac reserve. In cattle, peracute death
(Potter et al., 1984; Matsuoka et al., 1996). from monensin toxicity has not occurred. In fact, no
Laboratory and field studies have shown that there is a deaths have been recorded earlier than 3 days even after
threshold dose for individual ionophores below which no high-level (LD 50 ) toxic exposure (Potter et al., 1984).
adverse effects are observed. Safety studies have shown Blood-level studies suggest that horses, compared to
that consumption by horses of complete feed containing cattle, are not able to clear monensin rapidly from the
the maximum approved level of monensin for cattle is blood (Donoho, 1984). This was probably related to the
harmless. This is probably true for lasalocid and laidlo- oxidative efficiency of P450 demethylating enzymes in
mycin as well since there are no confirmed reports of ion- the liver. Working with liver microsomes from horses,
ophore toxicoses in horses used in cattle feedlots. pigs, broiler chicks, rats and cattle, Nebbia and associates
Results of acute toxicity studies with ionophores in (2001) found that horses had the lowest catalytic effi-
domestic animals indicate that the horse is the most sensi- ciency, which may explain the greater susceptibility of
tive species; the chicken least sensitive; and cattle inter- horses to the ionophore-induced toxicosis, relative to the
mediate. From studies in ponies given toxic doses of other species. However, not all levels of monensin are
monensin, Amend et al. (1981) found that the death pat- toxic to horses (Matsuoka et al., 1996). In a subchronic
tern from single, high toxic doses by gavage varied from feeding study in horses, all three horses provided a com-
peracute death in less than 24 h; acute death in 24 96 h; plete ration containing 330 ppm monensin (or 300 g/ton,
subacute death in 4 14 days; and chronic death after 14 10 times the maximum cleared level of monensin in com-
days. The latter is not a chronic toxicity by definition, but plete feeds for feedlot cattle) and one out of three horses
delayed death secondary to congestive heart failure given 121 ppm monensin died during the 1-month feeding
(CHF), a recognized consequence of ionophore toxicoses. period. However, three horses provided 33 ppm monensin