Page 1177 - Veterinary Toxicology, Basic and Clinical Principles, 3rd Edition
P. 1177
Water Quality and Contaminants Chapter | 80 1109
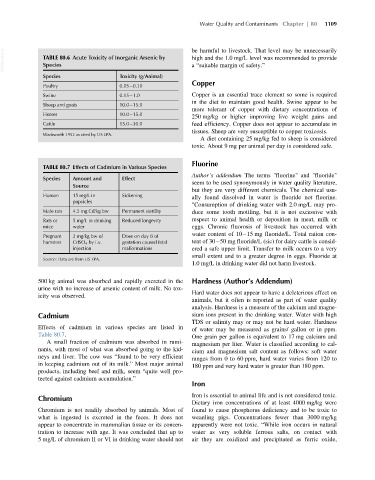
VetBooks.ir TABLE 80.6 Acute Toxicity of Inorganic Arsenic by be harmful to livestock. That level may be unnecessarily
high and the 1.0 mg/L level was recommended to provide
Species
a “suitable margin of safety.”
Species Toxicity (g/Animal)
Copper
Poultry 0.05 0.10
Swine 0.15 1.0 Copper is an essential trace element so some is required
in the diet to maintain good health. Swine appear to be
Sheep and goats 10.0 15.0
more tolerant of copper with dietary concentrations of
Horses 10.0 15.0
250 mg/kg or higher improving live weight gains and
Cattle 15.0 30.0 feed efficiency. Copper does not appear to accumulate in
tissues. Sheep are very susceptible to copper toxicosis.
Wadsworth 1952 as cited by US EPA.
A diet containing 25 mg/kg fed to sheep is considered
toxic. About 9 mg per animal per day is considered safe.
Fluorine
TABLE 80.7 Effects of Cadmium in Various Species
Author’s addendum The terms "fluorine" and "fluoride"
Species Amount and Effect
seem to be used synonymously in water quality literature,
Source
but they are very different chemicals. The chemical usu-
Human 15 mg/L in Sickening ally found dissolved in water is fluoride not fluorine.
popsicles
"Consumption of drinking water with 2.0 mg/L may pro-
Male rats 4.5 mg Cd/kg bw Permanent sterility duce some tooth mottling, but it is not excessive with
Rats or 5 mg/L in drinking Reduced longevity respect to animal health or deposition in meat, milk or
mice water eggs. Chronic fluorosis of livestock has occurred with
water content of 10 15 mg fluoride/L. Total ration con-
Pregnant 2 mg/kg bw of Dose on day 8 of
hamsters CdSO 4 by i.v. gestation caused fetal tent of 30 50 mg fluoride/L (sic) for dairy cattle is consid-
injection malformations ered a safe upper limit. Transfer to milk occurs to a very
small extent and to a greater degree in eggs. Fluoride at
Source: Data are from US EPA.
1.0 mg/L in drinking water did not harm livestock.
500 kg animal was absorbed and rapidly excreted in the Hardness (Author’s Addendum)
urine with no increase of arsenic content of milk. No tox-
Hard water does not appear to have a deleterious effect on
icity was observed.
animals, but it often is reported as part of water quality
analysis. Hardness is a measure of the calcium and magne-
Cadmium sium ions present in the drinking water. Water with high
TDS or salinity may or may not be hard water. Hardness
Effects of cadmium in various species are listed in of water may be measured as grains/ gallon or in ppm.
Table 80.7. One grain per gallon is equivalent to 17 mg calcium and
A small fraction of cadmium was absorbed in rumi- magnesium per liter. Water is classified according to cal-
nants, with most of what was absorbed going to the kid- cium and magnesium salt content as follows: soft water
neys and liver. The cow was “found to be very efficient ranges from 0 to 60 ppm, hard water varies from 120 to
in keeping cadmium out of its milk.” Most major animal 180 ppm and very hard water is greater than 180 ppm.
products, including beef and milk, seem “quite well pro-
tected against cadmium accumulation.”
Iron
Iron is essential to animal life and is not considered toxic.
Chromium
Dietary iron concentrations of at least 4000 mg/kg were
Chromium is not readily absorbed by animals. Most of found to cause phosphorus deficiency and to be toxic to
what is ingested is excreted in the feces. It does not weanling pigs. Concentrations fewer than 3000 mg/kg
appear to concentrate in mammalian tissue or its concen- apparently were not toxic. “While iron occurs in natural
tration to increase with age. It was concluded that up to water as very soluble ferrous salts, on contact with
5 mg/L of chromium II or VI in drinking water should not air they are oxidized and precipitated as ferric oxide,