Page 567 - Veterinary Toxicology, Basic and Clinical Principles, 3rd Edition
P. 567
534 SECTION | VI Insecticides
VetBooks.ir N N CI F or a single high dose than with a single low dose. The
long half-life (183 h in male and 245 h in female) of fipro-
nil in blood may reflect slow release of metabolites from
O N F fat (Woodward, 2012). The compounds identified in feces
S F and urine are the parent compound and sulfone, the amide
NH 2 CI derived from the nitrile group and a cleavage product of
F F
sulfone and its derivatives formed by further metabolism.
F Sulfone is the major metabolite in fat and tissues. In
essence, fipronil can be metabolized through three major
FIGURE 42.1 Chemical structure of fipronil.
pathways. Fipronil can be metabolized to fipronil sulfone
by oxidation at the sulfinyl moiety, to fipronil sulfide by
800
reduction at the sulfinyl moiety, and to fipronil amide by
hydrolysis of the cyano moiety (Wang et al., 2016).
700
In another pharmacokinetics study, rats received either
14
a single oral dose of a labeled compound ( C-fipronil-
600
desulfinyl) at 1 or 10 mg/kg body wt or daily oral doses
of unlabeled compound at 1 mg/kg body wt/day for
PPM values 500 14 days, followed by a single oral labeled dose. In
animals of both sexes, elimination of the radiolabeled
400
dose) than in the urine with all dosing regimens.
300 fipronil was much greater in the feces (46% 70% of the
Appreciable metabolic products were found in the tissues
200 1 week after treatment with the highest concentrations
being present in the fat and fatty tissues. The long half-
100
life in blood was 183 195 h and fat: plasma ratio of the
radiolabel compound was increased. Numerous metabo-
0
Day 0 Day 1 Day 8 Day 15 Day 22 Day 29 Day 36 lites or conjugates of fipronil desulfinyl were present in
Day after Frontline application the urine and feces. Only unchanged fipronil desulfinyl
was identified in the liver, fat, skin and body. There was
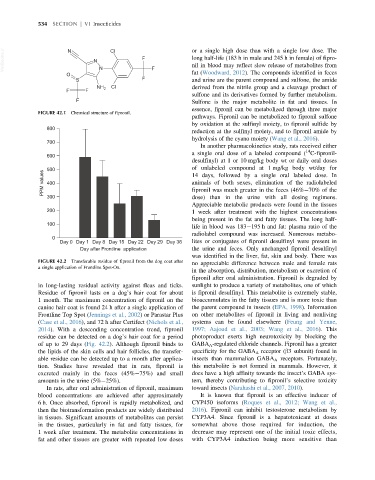
FIGURE 42.2 Transferable residue of fipronil from the dog coat after no appreciable difference between male and female rats
a single application of Frontline Spot-On.
in the absorption, distribution, metabolism or excretion of
fipronil after oral administration. Fipronil is degraded by
in long-lasting residual activity against fleas and ticks. sunlight to produce a variety of metabolites, one of which
Residue of fipronil lasts on a dog’s hair coat for about is fipronil desulfinyl. This metabolite is extremely stable,
1 month. The maximum concentration of fipronil on the bioaccumulates in the fatty tissues and is more toxic than
canine hair coat is found 24 h after a single application of the parent compound in insects (EPA, 1998). Information
Frontline Top Spot (Jennings et al., 2002) or Parastar Plus on other metabolites of fipronil in living and nonliving
(Case et al., 2016), and 72 h after Certifect (Nichols et al., systems can be found elsewhere (Feung and Yenne,
2014). With a descending concentration trend, fipronil 1997; Aajoud et al., 2003; Wang et al., 2016). This
residue can be detected on a dog’s hair coat for a period photoproduct exerts high neurotoxicity by blocking the
of up to 29 days (Fig. 42.2). Although fipronil binds to GABA A -regulated chloride channels. Fipronil has a greater
the lipids of the skin cells and hair follicles, the transfer- specificity for the GABA A receptor (β3 subunit) found in
able residue can be detected up to a month after applica- insects than mammalian GABA A receptors. Fortunately,
tion. Studies have revealed that in rats, fipronil is this metabolite is not formed in mammals. However, it
excreted mainly in the feces (45% 75%) and small does have a high affinity towards the insect’s GABA sys-
amounts in the urine (5% 25%). tem, thereby contributing to fipronil’s selective toxicity
In rats, after oral administration of fipronil, maximum toward insects (Narahashi et al., 2007, 2010).
blood concentrations are achieved after approximately It is known that fipronil is an effective inducer of
6 h. Once absorbed, fipronil is rapidly metabolized, and CYP450 isoforms (Roques et al., 2012; Wang et al.,
then the biotransformation products are widely distributed 2016). Fipronil can inhibit testosterone metabolism by
in tissues. Significant amounts of metabolites can persist CYP3A4. Since fipronil is a hepatotoxicant at doses
in the tissues, particularly in fat and fatty tissues, for somewhat above those required for induction, the
1 week after treatment. The metabolite concentrations in decrease may represent one of the initial toxic effects,
fat and other tissues are greater with repeated low doses with CYP3A4 induction being more sensitive than