Page 674 - Veterinary Toxicology, Basic and Clinical Principles, 3rd Edition
P. 674
Toxic Gases and Vapors Chapter | 48 639
VetBooks.ir Gaseous Ammonia has resulted in numerous serious incidents (Amshel et al.,
2000; Latenser and Lucktong, 2000; Lessenger, 2004;
Overview, Uses, and Sources of Exposure
Welch, 2006). Due to the unique chemical properties of
Within the veterinary context, ammonia (NH 3 ) is most anhydrous ammonia, it can exert extremely high pressures
commonly encountered anywhere decaying organic matter even at relatively low temperatures; thus, specialized con-
is present, particularly urine and feces (Roney et al., tainers and equipment are required for safe handling of
2004). In this context, it is ubiquitous within intensive the gas. Attempts at anhydrous ammonia theft often
animal production facilities. Swine and poultry production involve the use of propane tanks that are not designed to
facilities are notorious for containing toxic levels of handle the physicochemical properties of anhydrous
ammonia that are often higher than acceptable human ammonia, and the risk of explosion of these types of con-
threshold limit values (Sigurdarson et al., 2004; Davis and tainers is high. Veterinarians and farmers should be
Morishita, 2005; McDonnell et al., 2008). Ammonia is extremely cautious around apparently empty propane
lighter than air and will thus tend to rise from manure pits tanks that have blue- or green-discolored valves or if the
(Roney et al., 2004). Ammonia is still used as a refriger- tanks have frost on them. Anhydrous ammonia may have
ant, and leaks in such systems remain a common cause of been inappropriately stored in such containers, and the
serious incidents. The burning of nylon, silk, wood, and container’s brass, copper, or galvanized valve fittings may
melamine also results in considerable production of NH 3 . be compromised.
NH 3 is a major component of many common household Liquid anhydrous ammonia expands to many times its
cleaning and bleaching products, and the mixing of these original volume when released into air and forms large,
products with those that contain chlorine results in the lib- highly dangerous vapor clouds. Aerosolized liquid anhy-
eration of chloramines, which are highly irritant and drous ammonia may behave as a dense gas and accumu-
potentially dangerous (Pascuzzi and Storrow, 1998). late in low-lying spaces, even though it is normally
High-pressure anhydrous ammonia gas is a commonly lighter than air.
used fertilizer in some locations because it is often the
cheapest source of nitrogen. Errors in handling (often asso-
ciated with its illicit use for the production of methamphet- Toxic Dose
amine) and leakage represent a substantial risk (George The concentration of NH 3 in well-ventilated animal pro-
et al., 2000; Fitzgerald and Flood, 2006; Welch, 2006). duction facilities should remain below 30 ppm. The human
Anhydrous ammonia is an important component in the odor threshold is approximately 10 ppm. The human dose
manufacture of methamphetamines (Bloom et al., 2008). response to NH 3 is shown in Table 48.3. Adverse effects
Theft of anhydrous ammonia from farms is common and occur at concentrations greater than approximately 75 ppm.
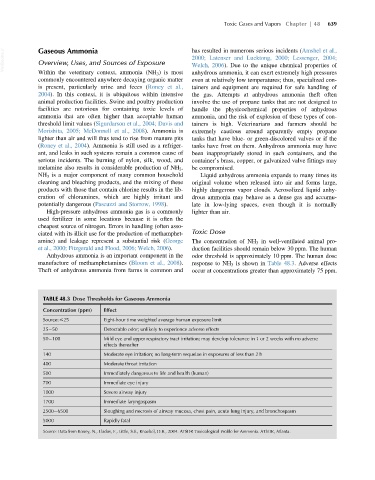
TABLE 48.3 Dose Thresholds for Gaseous Ammonia
Concentration (ppm) Effect
Source: #25 Eight-hour time weighted average human exposure limit
25 50 Detectable odor; unlikely to experience adverse effects
50 100 Mild eye and upper respiratory tract irritation; may develop tolerance in 1 or 2 weeks with no adverse
effects thereafter
140 Moderate eye irritation; no long-term sequelae in exposures of less than 2 h
400 Moderate throat irritation
500 Immediately dangerous to life and health (human)
700 Immediate eye injury
1000 Severe airway injury
1700 Immediate laryngospasm
2500 6500 Sloughing and necrosis of airway mucosa, chest pain, acute lung injury, and bronchospasm
5000 Rapidly fatal
Source: Data from Roney, N., Llados, F., Little, S.S., Knaebel, D.B., 2004. ATSDR Toxicological Profile for Ammonia. ATSDR, Atlanta.