Page 863 - Veterinary Toxicology, Basic and Clinical Principles, 3rd Edition
P. 863
Phytoestrogens Chapter | 60 821
VetBooks.ir
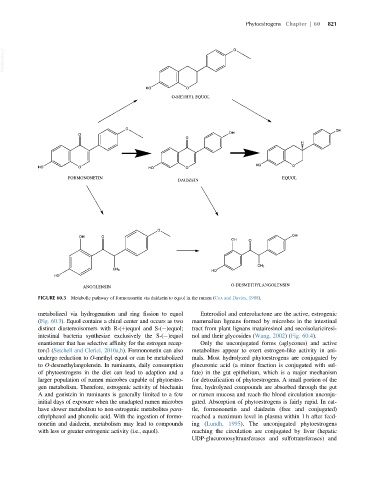
FIGURE 60.3 Metabolic pathway of formononetin via daidzein to equol in the rumen (Cox and Davies, 1988).
metabolized via hydrogenation and ring fission to equol Enterodiol and enterolactone are the active, estrogenic
(Fig. 60.3). Equol contains a chiral center and occurs as two mammalian lignans formed by microbes in the intestinal
distinct diastereoisomers with R-(1)equol and S-( )equol; tract from plant lignans matairesinol and secoisolariciresi-
intestinal bacteria synthesize exclusively the S-( )equol nol and their glycosides (Wang, 2002)(Fig. 60.4).
enantiomer that has selective affinity for the estrogen recep- Only the unconjugated forms (aglycones) and active
tor-β (Setchell and Clerici, 2010a,b). Formononetin can also metabolites appear to exert estrogen-like activity in ani-
undergo reduction to O-methyl equol or can be metabolized mals. Most hydrolyzed phytoestrogens are conjugated by
to O-desmethylangolensin. In ruminants, daily consumption glucuronic acid (a minor fraction is conjugated with sul-
of phytoestrogens in the diet can lead to adaption and a fate) in the gut epithelium, which is a major mechanism
larger population of rumen microbes capable of phytoestro- for detoxification of phytoestrogens. A small portion of the
gen metabolism. Therefore, estrogenic activity of biochanin free, hydrolyzed compounds are absorbed through the gut
A and genistein in ruminants is generally limited to a few or rumen mucosa and reach the blood circulation unconju-
initial days of exposure when the unadapted rumen microbes gated. Absorption of phytoestrogens is fairly rapid. In cat-
have slower metabolism to non-estrogenic metabolites para- tle, formononetin and daidzein (free and conjugated)
ethylphenol and phenolic acid. With the ingestion of formo- reached a maximum level in plasma within 1 h after feed-
nonetin and daidzein, metabolism may lead to compounds ing (Lundh, 1995). The unconjugated phytoestrogens
with less or greater estrogenic activity (i.e., equol). reaching the circulation are conjugated by liver (hepatic
UDP-glucuronosyltransferases and sulfotransferases) and