Page 867 - Veterinary Toxicology, Basic and Clinical Principles, 3rd Edition
P. 867
Phytoestrogens Chapter | 60 825
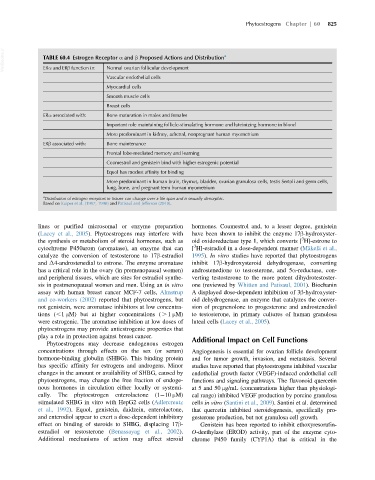
VetBooks.ir TABLE 60.4 Estrogen Receptor α and β Proposed Actions and Distribution a
Normal ovarian follicular development
ERα and ERβ function in:
Vascular endothelial cells
Myocardial cells
Smooth muscle cells
Breast cells
ERα associated with: Bone maturation in males and females
Important role maintaining follicle-stimulating hormone and luteinizing hormone in blood
More predominant in kidney, adrenal, nonpregnant human myometrium
ERβ associated with: Bone maintenance
Frontal lobe-mediated memory and learning
Coumestrol and genistein bind with higher estrogenic potential
Equol has modest affinity for binding
More predominant in human brain, thymus, bladder, ovarian granulosa cells, testis Sertoli and germ cells,
lung, bone, and pregnant term human myometrium
a
Distribution of estrogen receptors in tissues can change over a life span and is sexually dimorphic.
Based on Kuiper et al. (1997, 1998) and Patisaul and Jefferson (2010).
lines or purified microsomal or enzyme preparation hormones. Coumestrol and, to a lesser degree, genistein
(Lacey et al., 2005). Phytoestrogens may interfere with have been shown to inhibit the enzyme 17β-hydroxyster-
3
the synthesis or metabolism of steroid hormones, such as oid oxidoreductase type 1, which converts [ H]-estrone to
3
cytochrome P450arom (aromatase), an enzyme that can [ H]-estradiol in a dose-dependent manner (Ma ¨kela ¨ et al.,
catalyze the conversion of testosterone to 17β-estradiol 1995). In vitro studies have reported that phytoestrogens
and Δ4-androstenedial to estrone. The enzyme aromatase inhibit 17β-hydroxysteroid dehydrogenase, converting
has a critical role in the ovary (in premenopausal women) androstenedione to testosterone, and 5α-reductase, con-
and peripheral tissues, which are sites for estradiol synthe- verting testosterone to the more potent dihydrotestoster-
sis in postmenopausal women and men. Using an in vitro one (reviewed by Whitten and Patisaul, 2001). Biochanin
assay with human breast cancer MCF-7 cells, Almstrup A displayed dose-dependent inhibition of 3β-hydroxyster-
and co-workers (2002) reported that phytoestrogens, but oid dehydrogenase, an enzyme that catalyzes the conver-
not genistein, were aromatase inhibitors at low concentra- sion of pregnenolone to progesterone and androstenediol
tions (,1 μM) but at higher concentrations ( . 1 μM) to testosterone, in primary cultures of human granulosa
were estrogenic. The aromatase inhibition at low doses of luteal cells (Lacey et al., 2005).
phytoestrogens may provide antiestrogenic properties that
play a role in protection against breast cancer. Additional Impact on Cell Functions
Phytoestrogens may decrease endogenous estrogen
concentrations through effects on the sex (or serum) Angiogenesis is essential for ovarian follicle development
hormone-binding globulin (SHBG). This binding protein and for tumor growth, invasion, and metastasis. Several
has specific affinity for estrogens and androgens. Minor studies have reported that phytoestrogens inhibited vascular
changes in the amount or availability of SHBG, caused by endothelial growth factor (VEGF)-induced endothelial cell
phytoestrogens, may change the free fraction of endoge- functions and signaling pathways. The flavonoid quercetin
nous hormones in circulation either locally or systemi- at 5 and 50 μg/mL (concentrations higher than physiologi-
cally. The phytoestrogen enterolactone (1 10 μM) cal range) inhibited VEGF production by porcine granulosa
stimulated SHBG in vitro with HepG2 cells (Adlercreutz cells in vitro (Santini et al., 2009). Santini et al. determined
et al., 1992). Equol, genistein, daidzein, enterolactone, that quercetin inhibited steroidogenesis, specifically pro-
and enterodiol appear to exert a dose-dependent inhibitory gesterone production, but not granulosa cell growth.
effect on binding of steroids to SHBG, displacing 17β- Genistein has been reported to inhibit ethoxyresorufin-
estradiol or testosterone (Benassayag et al., 2002). O-deethylase (EROD) activity, part of the enzyme cyto-
Additional mechanisms of action may affect steroid chrome P450 family (CYP1A) that is critical in the