Page 711 - Small Animal Internal Medicine, 6th Edition
P. 711
CHAPTER 40 Glomerular Disease 683
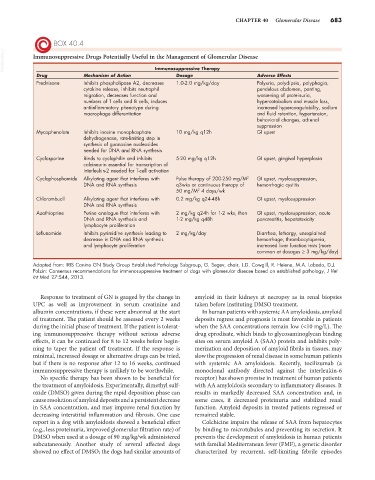
BOX 40.4
VetBooks.ir Immunosuppressive Drugs Potentially Useful in the Management of Glomerular Disease
Drug Mechanism of Action Immunosuppressive Therapy Adverse Effects
Dosage
Prednisone Inhibits phospholipase A2, decreases 1.0-2.0 mg/kg/day Polyuria, polydipsia, polyphagia,
cytokine release, inhibits neutrophil pendulous abdomen, panting,
migration, decreases function and worsening of proteinuria,
numbers of T cells and B cells, induces hypercatabolism and muscle loss,
antiinflammatory phenotype during increased hypercoagulability, sodium
macrophage differentiation and fluid retention, hypertension,
behavioral changes, adrenal
suppression
Mycophenolate Inhibits inosine monophosphate 10 mg/kg q12h GI upset
dehydrogenase, rate-limiting step in
synthesis of guanosine nucleosides
needed for DNA and RNA synthesis
Cyclosporine Binds to cyclophilin and inhibits 5-20 mg/kg q12h GI upset, gingival hyperplasia
calcineurin essential for transcription of
interleukin-2 needed for T-cell activation
2
Cyclophosphamide Alkylating agent that interferes with Pulse therapy of 200-250 mg/M GI upset, myelosuppression,
DNA and RNA synthesis q3wks or continuous therapy of hemorrhagic cystitis
2
50 mg/M 4 days/wk
Chlorambucil Alkylating agent that interferes with 0.2 mg/kg q24-48h GI upset, myelosuppression
DNA and RNA synthesis
Azathioprine Purine analogue that interferes with 2 mg/kg q24h for 1-2 wks, then GI upset, myelosuppression, acute
DNA and RNA synthesis and 1-2 mg/kg q48h pancreatitis, hepatotoxicity
lymphocyte proliferation
Leflunomide Inhibits pyrimidine synthesis leading to 2 mg/kg/day Diarrhea, lethargy, unexplained
decrease in DNA and RNA synthesis hemorrhage, thrombocytopenia,
and lymphocyte proliferation increased liver function tests (more
common at dosages ≥ 3 mg/kg/day)
Adapted from: IRIS Canine GN Study Group Established Pathology Subgroup, G. Segev, chair, L.D. Cowgill, R. Heiene, M.A. Labado, D.J.
Polzin: Consensus recommendations for immunosuppressive treatment of dogs with glomerular disease based on established pathology, J Vet
Int Med 27:S44, 2013.
Response to treatment of GN is gauged by the change in amyloid in their kidneys at necropsy as in renal biopsies
UPC as well as improvement in serum creatinine and taken before instituting DMSO treatment.
albumin concentrations, if these were abnormal at the start In human patients with systemic AA amyloidosis, amyloid
of treatment. The patient should be assessed every 2 weeks deposits regress and prognosis is most favorable in patients
during the initial phase of treatment. If the patient is tolerat- when the SAA concentrations remain low (<10 mg/L). The
ing immunosuppressive therapy without serious adverse drug eprodisate, which binds to glycosaminoglycan binding
effects, it can be continued for 8 to 12 weeks before begin- sites on serum amyloid A (SAA) protein and inhibits poly-
ning to taper the patient off treatment. If the response is merization and deposition of amyloid fibrils in tissues, may
minimal, increased dosage or alternative drugs can be tried, slow the progression of renal disease in some human patients
but if there is no response after 12 to 16 weeks, continued with systemic AA amyloidosis. Recently, tocilizumab (a
immunosuppressive therapy is unlikely to be worthwhile. monoclonal antibody directed against the interleukin-6
No specific therapy has been shown to be beneficial for receptor) has shown promise in treatment of human patients
the treatment of amyloidosis. Experimentally, dimethyl sulf- with AA amyloidosis secondary to inflammatory diseases. It
oxide (DMSO) given during the rapid deposition phase can results in markedly decreased SAA concentration and, in
cause resolution of amyloid deposits and a persistent decrease some cases, it decreased proteinuria and stabilized renal
in SAA concentration, and may improve renal function by function. Amyloid deposits in treated patients regressed or
decreasing interstitial inflammation and fibrosis. One case remained stable.
report in a dog with amyloidosis showed a beneficial effect Colchicine impairs the release of SAA from hepatocytes
(e.g., less proteinuria, improved glomerular filtration rate) of by binding to microtubules and preventing its secretion. It
DMSO when used at a dosage of 90 mg/kg/wk administered prevents the development of amyloidosis in human patients
subcutaneously. Another study of several affected dogs with familial Mediterranean fever (FMF), a genetic disorder
showed no effect of DMSO; the dogs had similar amounts of characterized by recurrent, self-limiting febrile episodes