Page 104 - Veterinary Immunology, 10th Edition
P. 104
VetBooks.ir
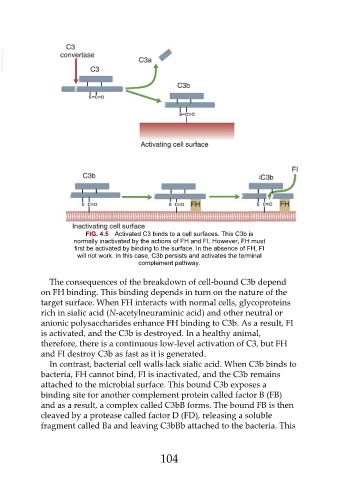
FIG. 4.5 Activated C3 binds to a cell surfaces. This C3b is
normally inactivated by the actions of FH and FI. However, FH must
first be activated by binding to the surface. In the absence of FH, FI
will not work. In this case, C3b persists and activates the terminal
complement pathway.
The consequences of the breakdown of cell-bound C3b depend
on FH binding. This binding depends in turn on the nature of the
target surface. When FH interacts with normal cells, glycoproteins
rich in sialic acid (N-acetylneuraminic acid) and other neutral or
anionic polysaccharides enhance FH binding to C3b. As a result, FI
is activated, and the C3b is destroyed. In a healthy animal,
therefore, there is a continuous low-level activation of C3, but FH
and FI destroy C3b as fast as it is generated.
In contrast, bacterial cell walls lack sialic acid. When C3b binds to
bacteria, FH cannot bind, FI is inactivated, and the C3b remains
attached to the microbial surface. This bound C3b exposes a
binding site for another complement protein called factor B (FB)
and as a result, a complex called C3bB forms. The bound FB is then
cleaved by a protease called factor D (FD), releasing a soluble
fragment called Ba and leaving C3bBb attached to the bacteria. This
104