Page 107 - Veterinary Immunology, 10th Edition
P. 107
VetBooks.ir
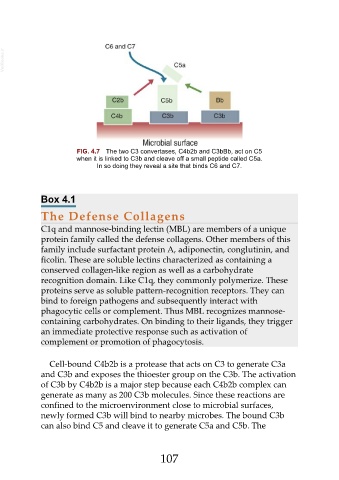
FIG. 4.7 The two C3 convertases, C4b2b and C3bBb, act on C5
when it is linked to C3b and cleave off a small peptide called C5a.
In so doing they reveal a site that binds C6 and C7.
Box 4.1
The Defense Collagens
C1q and mannose-binding lectin (MBL) are members of a unique
protein family called the defense collagens. Other members of this
family include surfactant protein A, adiponectin, conglutinin, and
ficolin. These are soluble lectins characterized as containing a
conserved collagen-like region as well as a carbohydrate
recognition domain. Like C1q, they commonly polymerize. These
proteins serve as soluble pattern-recognition receptors. They can
bind to foreign pathogens and subsequently interact with
phagocytic cells or complement. Thus MBL recognizes mannose-
containing carbohydrates. On binding to their ligands, they trigger
an immediate protective response such as activation of
complement or promotion of phagocytosis.
Cell-bound C4b2b is a protease that acts on C3 to generate C3a
and C3b and exposes the thioester group on the C3b. The activation
of C3b by C4b2b is a major step because each C4b2b complex can
generate as many as 200 C3b molecules. Since these reactions are
confined to the microenvironment close to microbial surfaces,
newly formed C3b will bind to nearby microbes. The bound C3b
can also bind C5 and cleave it to generate C5a and C5b. The
107