Page 234 - Natural Antioxidants, Applications in Foods of Animal Origin
P. 234
Methods and Their Applications for Measuring 213
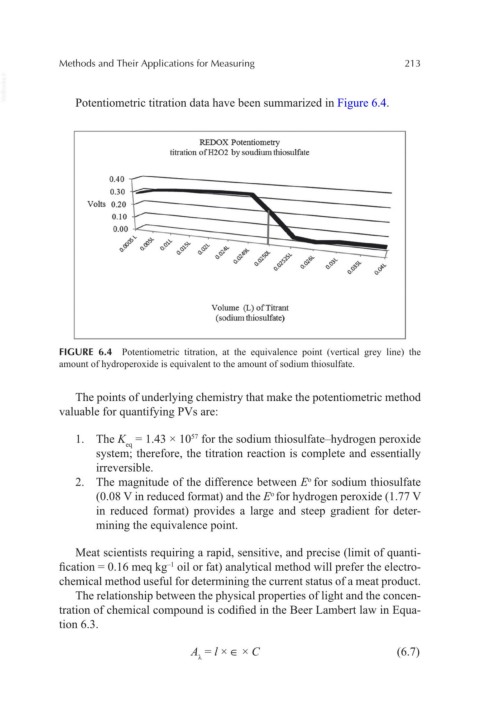
VetBooks.ir Potentiometric titration data have been summarized in Figure 6.4.
REDOX Potentiometry
titration ofH202 by soudium thiosulfate
0.40 1:::::::::===-------------
0.30 t:=::=:::::~!!!!!!!l'~~=========
Volts 0.20
0.10
0.00
Volume (L) ofTitrant
(sodium thiosulfate)
FIGURE 6.4 Potentiometric titration, at the equivalence point (vertical grey line) the
amount of hydroperoxide is equivalent to the amount of sodium thiosulfate.
The points of underlying chemistry that make the potentiometric method
valuable for quantifying PVs are:
1. The K = 1.43 × 10 for the sodium thiosulfate–hydrogen peroxide
57
eq
system; therefore, the titration reaction is complete and essentially
irreversible.
2. The magnitude of the difference between E for sodium thiosulfate
o
(0.08 V in reduced format) and the E for hydrogen peroxide (1.77 V
o
in reduced format) provides a large and steep gradient for deter-
mining the equivalence point.
Meat scientists requiring a rapid, sensitive, and precise (limit of quanti-
fication = 0.16 meq kg oil or fat) analytical method will prefer the electro-
–1
chemical method useful for determining the current status of a meat product.
The relationship between the physical properties of light and the concen-
tration of chemical compound is codified in the Beer Lambert law in Equa-
tion 6.3.
A = l × ∈ × C (6.7)
λ