Page 238 - Natural Antioxidants, Applications in Foods of Animal Origin
P. 238
Methods and Their Applications for Measuring 217
VetBooks.ir meet specification limits must be “re-worked,” discounted in value, or worse
case thrown out. However, the data can be used to frame relevant ques-
tions for managing oxidation. For example, is the specification realistic or
could the process be improved in the selection of raw material quality and/
or production methods.
6.2.2 ANISIDINE VALUES (p-AV)
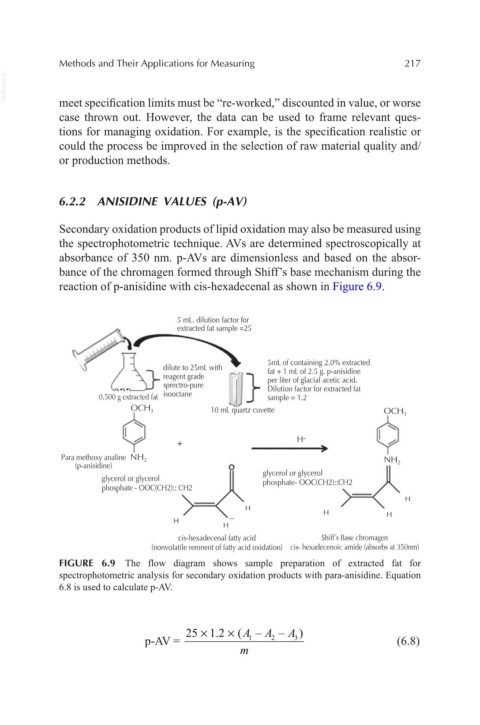
Secondary oxidation products of lipid oxidation may also be measured using
the spectrophotometric technique. AVs are determined spectroscopically at
absorbance of 350 nm. p-AVs are dimensionless and based on the absor-
bance of the chromagen formed through Shiff’s base mechanism during the
reaction of p-anisidine with cis-hexadecenal as shown in Figure 6.9.
) ml. dilution factor for
~ " "" "" ful " mpleo25
fc
mlo
~ %extracted
)
ont
inin
g
2.0
a
dilute to 2Sml with fat+ 1 ml of 2.) g. p-anisidine
reagent grade ] I } per liter of glacial acetic acid.
& sprectro-pure Dilution factor for extracted fat
AH3 10 ml quartz cuvette ¢"'
0.500 g ~xlracled fal isooctane sample = 1 2
.
>==!
~ + H-
Para methoxJ analine NH 2 NH 2
(p-anisi ine) ~
glycerol or glycerol glycerol or glycerol
phosphate- OOC(CH2)::CH2--
phosphate - OOC(CH2):: CH2 -- H
H
H H
H H -
cis-hexadecenal fatty acid Shiff's Base chromagen
(nonvolatile remnent of fatty acid oxidation) cis- hexadecenoic amide (absorbs al 350nm)
FIGURE 6.9 The flow diagram shows sample preparation of extracted fat for
spectrophotometric analysis for secondary oxidation products with para-anisidine. Equation
6.8 is used to calculate p-AV.
25 1.2 (A – A – A )
×
×
p-AV = 1 2 3 (6.8)
m