Page 27 - Natural Antioxidants, Applications in Foods of Animal Origin
P. 27
6 Natural Antioxidants: Applications in Foods of Animal Origin
VetBooks.ir Renerre, 2000; Fig. 1.1). Oxidation is initiated by radicals present in living
organisms (e.g., hydroperoxide, hydroxide, peroxide, alcoxy, and alkyl)
or by thermal or photochemical homolytic cleavage of an R-H bond. The
oxidation activation energy and reaction rate at this stage depend on the type
of initiator and the number of unsaturated bonds in the substrate. The disso-
ciation energy of the C-H bonds in saturated fatty acid depend on the length
of the fatty acid carbon chain and is similar in fatty acid, their esters and in
triacylglycerols (Litwinienko et al., 1999). In unsaturated acids, the weakest
C-H bond is found in the bis-allylic position. The activation energies are
75, 88, and 100 kcal/mol for bis-allylic, allylic, and methylene hydrogens,
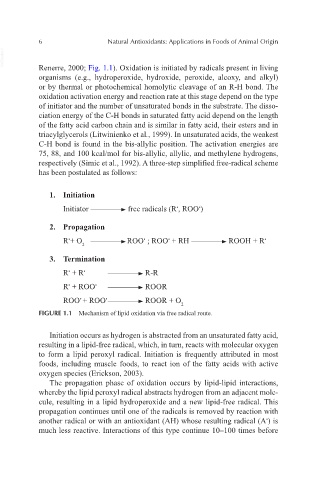
respectively (Simic et al., 1992). A three-step simplified free-radical scheme
has been postulated as follows:
1. Initiation
Initiator free radicals (R , ROO )
•
•
2. Propagation
R + O ROO ; ROO + RH ROOH + R •
•
•
•
2
3. Termination
R + R R-R
•
•
R + ROO ROOR
•
•
ROO + ROO ROOR + O
•
•
2
FIGURE 1.1 Mechanism of lipid oxidation via free radical route.
Initiation occurs as hydrogen is abstracted from an unsaturated fatty acid,
resulting in a lipid-free radical, which, in turn, reacts with molecular oxygen
to form a lipid peroxyl radical. Initiation is frequently attributed in most
foods, including muscle foods, to react ion of the fatty acids with active
oxygen species (Erickson, 2003).
The propagation phase of oxidation occurs by lipid-lipid interactions,
whereby the lipid peroxyl radical abstracts hydrogen from an adjacent mole-
cule, resulting in a lipid hydroperoxide and a new lipid-free radical. This
propagation continues until one of the radicals is removed by reaction with
another radical or with an antioxidant (AH) whose resulting radical (A ) is
•
much less reactive. Interactions of this type continue 10–100 times before