Page 73 - Natural Antioxidants, Applications in Foods of Animal Origin
P. 73
52 Natural Antioxidants: Applications in Foods of Animal Origin
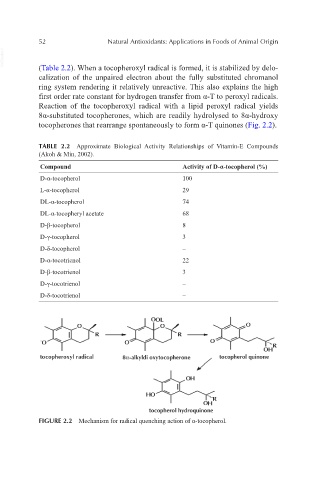
VetBooks.ir (Table 2.2). When a tocopheroxyl radical is formed, it is stabilized by delo-
calization of the unpaired electron about the fully substituted chromanol
ring system rendering it relatively unreactive. This also explains the high
first order rate constant for hydrogen transfer from α-T to peroxyl radicals.
Reaction of the tocopheroxyl radical with a lipid peroxyl radical yields
8α-substituted tocopherones, which are readily hydrolysed to 8α-hydroxy
tocopherones that rearrange spontaneously to form α-T quinones (Fig. 2.2).
TABLE 2.2 Approximate Biological Activity Relationships of Vitamin-E Compounds
(Akoh & Min, 2002).
Compound Activity of D-α-tocopherol (%)
D-α-tocopherol 100
L-α-tocopherol 29
DL-α-tocopherol 74
DL-α-tocopheryl acetate 68
D-β-tocopherol 8
D-γ-tocopherol 3
D-δ-tocopherol –
D-α-tocotrienol 22
D-β-tocotrienol 3
D-γ-tocotrienol –
D-δ-tocotrienol –
tocopheroxyl radical 8a-alkyldi oxytocopherone / tocopherol quinone
HO
tocopherol hydroquinone
FIGURE 2.2 Mechanism for radical quenching action of α-tocopherol.