Page 79 - Natural Antioxidants, Applications in Foods of Animal Origin
P. 79
58 Natural Antioxidants: Applications in Foods of Animal Origin
VetBooks.ir
HO OH
OH
0
OH
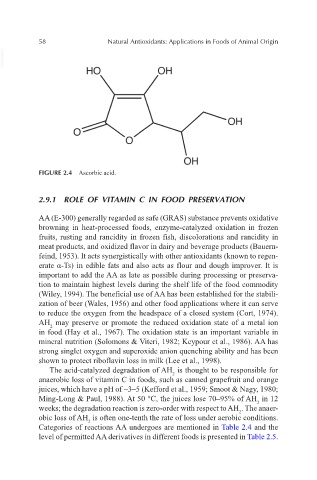
FIGURE 2.4 Ascorbic acid.
2.9.1 ROLE OF VITAMIN C IN FOOD PRESERVATION
AA (E-300) generally regarded as safe (GRAS) substance prevents oxidative
browning in heat-processed foods, enzyme-catalyzed oxidation in frozen
fruits, rusting and rancidity in frozen fish, discolorations and rancidity in
meat products, and oxidized flavor in dairy and beverage products (Bauern-
feind, 1953). It acts synergistically with other antioxidants (known to regen-
erate α-Ts) in edible fats and also acts as flour and dough improver. It is
important to add the AA as late as possible during processing or preserva-
tion to maintain highest levels during the shelf life of the food commodity
(Wiley, 1994). The beneficial use of AA has been established for the stabili-
zation of beer (Wales, 1956) and other food applications where it can serve
to reduce the oxygen from the headspace of a closed system (Cort, 1974).
AH may preserve or promote the reduced oxidation state of a metal ion
2
in food (Hay et al., 1967). The oxidation state is an important variable in
mineral nutrition (Solomons & Viteri, 1982; Keypour et al., 1986). AA has
strong singlet oxygen and superoxide anion quenching ability and has been
shown to protect riboflavin loss in milk (Lee et al., 1998).
The acid-catalyzed degradation of AH is thought to be responsible for
2
anaerobic loss of vitamin C in foods, such as canned grapefruit and orange
juices, which have a pH of ~3–5 (Kefford et al., 1959; Smoot & Nagy, 1980;
Ming-Long & Paul, 1988). At 50 °C, the juices lose 70–95% of AH in 12
2
weeks; the degradation reaction is zero-order with respect to AH . The anaer-
2
obic loss of AH is often one-tenth the rate of loss under aerobic conditions.
2
Categories of reactions AA undergoes are mentioned in Table 2.4 and the
level of permitted AA derivatives in different foods is presented in Table 2.5.