Page 1231 - Clinical Small Animal Internal Medicine
P. 1231
125 Management of Chronic Kidney Disease 1169
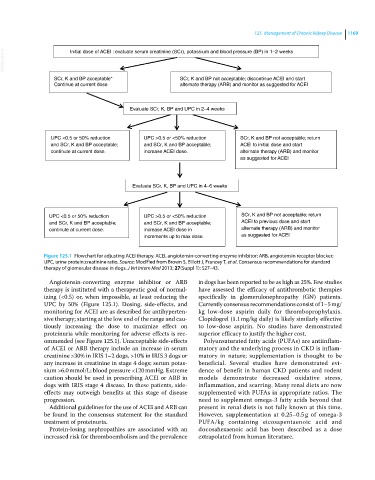
VetBooks.ir Initial dose of ACEI : evaluate serum creatinine (SCr), potassium and blood pressure (BP) in 1–2 weeks
SCr, K and BP acceptable* SCr, K and BP not acceptable; discontinue ACEI and start
Continue at current dose alternate therapy (ARB) and monitor as suggested for ACEI
Evaluate SCr, K, BP and UPC in 2–4 weeks
UPC <0.5 or 50% reduction UPC >0.5 or <50% reduction SCr, K and BP not acceptable; return
and SCr, K and BP acceptable; and SCr, K and BP acceptable; ACEI to initial dose and start
continute at current dose. increase ACEI dose. alternate therapy (ARB) and monitor
as suggested for ACEI
Evaluate SCr, K, BP and UPC in 4–6 weeks
UPC <0.5 or 50% reduction UPC >0.5 or <50% reduction SCr, K and BP not acceptable; return
and SCr, K and BP acceptable; and SCr, K and BP acceptable; ACEI to previous dose and start
continute at current dose. increase ACEI dose in alternate therapy (ARB) and monitor
increments up to max dose. as suggested for ACEI
Figure 125.1 Flowchart for adjusting ACEI therapy. ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker;
UPC, urine protein:creatinine ratio. Source: Modified from Brown S, Elliott J, Francey T, et al. Consensus recommendations for standard
therapy of glomerular disease in dogs. J Vet Intern Med 2013; 27(Suppl 1): S27–43.
Angiotensin‐converting enzyme inhibitor or ARB in dogs has been reported to be as high as 25%. Few studies
therapy is instituted with a therapeutic goal of normal- have assessed the efficacy of antithrombotic therapies
izing (<0.5) or, when impossible, at least reducing the specifically in glomerulonephropathy (GN) patients.
UPC by 50% (Figure 125.1). Dosing, side‐effects, and Currently consensus recommendations consist of 1–5 mg/
monitoring for ACEI are as described for antihyperten- kg low‐dose aspirin daily for thromboprophylaxis.
sive therapy; starting at the low end of the range and cau- Clopidogrel (1.1 mg/kg daily) is likely similarly effective
tiously increasing the dose to maximize effect on to low‐dose aspirin. No studies have demonstrated
proteinuria while monitoring for adverse effects is rec- superior efficacy to justify the higher cost.
ommended (see Figure 125.1). Unacceptable side‐effects Polyunsaturated fatty acids (PUFAs) are antiinflam-
of ACEI or ARB therapy include an increase in serum matory and the underlying process in CKD is inflam-
creatinine >30% in IRIS 1–2 dogs, >10% in IRIS 3 dogs or matory in nature; supplementation is thought to be
any increase in creatinine in stage 4 dogs; serum potas- beneficial. Several studies have demonstrated evi-
sium >6.0 mmol/L; blood pressure <120 mmHg. Extreme dence of benefit in human CKD patients and rodent
caution should be used in prescribing ACEI or ARB in models demonstrate decreased oxidative stress,
dogs with IRIS stage 4 disease. In these patients, side‐ inflammation, and scarring. Many renal diets are now
effects may outweigh benefits at this stage of disease supplemented with PUFAs in appropriate ratios. The
progression. need to supplement omega‐3 fatty acids beyond that
Additional guidelines for the use of ACEI and ARB can present in renal diets is not fully known at this time.
be found in the consensus statement for the standard However, supplementation at 0.25–0.5 g of omega‐3
treatment of proteinuria. PUFA/kg containing eicosapentaenoic acid and
Protein‐losing nephropathies are associated with an docosahexaenoic acid has been described as a dose
increased risk for thromboembolism and the prevalence extrapolated from human literature.