Page 309 - The Toxicology of Fishes
P. 309
Reactive Oxygen Species and Oxidative Stress 289
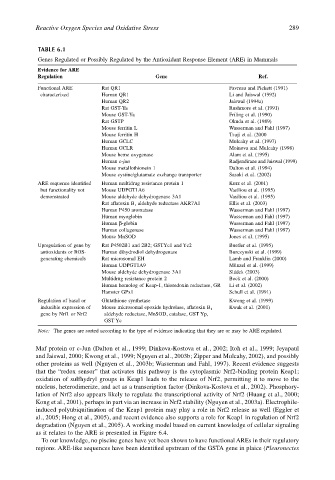
TABLE 6.1
Genes Regulated or Possibly Regulated by the Antioxidant Response Element (ARE) in Mammals
Evidence for ARE
Regulation Gene Ref.
Functional ARE Rat QR1 Favreau and Pickett (1991)
characterized Human QR1 Li and Jaiswal (1992)
Human QR2 Jaiswal (1994a)
Rat GST-Ya Rushmore et al. (1991)
Mouse GST-Ya Friling et al. (1990)
Rat GSTP Okuda et al. (1989)
Mouse ferritin L Wasserman and Fahl (1997)
Mouse ferritin H Tsuji et al. (2000
Human GCLC Mulcahy et al. (1997)
Human GCLR Moinova and Mulcahy (1998)
Mouse heme oxygenase Alam et al. (1995)
Human c-jun Radjendirane and Jaiswal (1999)
Mouse metallothionein 1 Dalton et al. (1994)
Mouse cystine/glutamate exchange transporter Sasaki et al. (2002)
ARE sequence identified Human multidrug resistance protein 1 Kurz et al. (2001)
but functionality not Mouse UDPGT1A6 Vasiliou et al. (1995)
demonstrated Mouse aldehyde dehydrogenase 3A1 Vasiliou et al. (1995)
Rat aflatoxin B 1 aldehyde reductase AKR7A1 Ellis et al. (2003)
Human P450 aromatase Wasserman and Fahl (1997)
Human myoglobin Wasserman and Fahl (1997)
Human β-globin Wasserman and Fahl (1997)
Human collagenase Wasserman and Fahl (1997)
Mouse MnSOD Jones et al. (1995)
Upregulation of gene by Rat P4502B1 and 2B2; GSTYc1 and Yc2 Buetler et al. (1995)
antioxidants or ROS- Human dihydrodiol dehydrogenase Burczynski et al. (1999)
generating chemicals Rat microsomal EH Lamb and Franklin (2000)
Human UDPGT1A9 Münzel et al. (1999)
Mouse aldehyde dehydrogenase 3A1 Sládek (2003)
Multidrug resistance protein 2 Bock et al. (2000)
Human homolog of Keap-1, thioredoxin reductase, GR Li et al. (2002)
Hamster GPx1 Schull et al. (1991)
Regulation of basal or Glutathione synthetase Kwong et al. (1999)
inducible expression of Mouse microsomal epoxide hydrolase, aflatoxin B 1 Kwak et al. (2001)
gene by Nrf1 or Nrf2 aldehyde reductase, MnSOD, catalase, GST Yp,
GST Yc
Note: The genes are sorted according to the type of evidence indicating that they are or may be ARE regulated.
Maf protein or c-Jun (Dalton et al., 1999; Dinkova-Kostova et al., 2002; Itoh et al., 1999; Jeyapaul
and Jaiswal, 2000; Kwong et al., 1999; Nguyen et al., 2003b; Zipper and Mulcahy, 2002), and possibly
other proteins as well (Nguyen et al., 2003b; Wasserman and Fahl, 1997). Recent evidence suggests
that the “redox sensor” that activates this pathway is the cytoplasmic Nrf2-binding protein Keap1;
oxidation of sulfhydryl groups in Keap1 leads to the release of Nrf2, permitting it to move to the
nucleus, heterodimerize, and act as a transcription factor (Dinkova-Kostova et al., 2002). Phosphory-
lation of Nrf2 also appears likely to regulate the transcriptional activity of Nrf2 (Huang et al., 2000;
Kong et al., 2001), perhaps in part via an increase in Nrf2 stability (Nguyen et al., 2003a). Electrophile-
induced polyubiquitination of the Keap1 protein may play a role in Nrf2 release as well (Eggler et
al., 2005; Hong et al., 2005), and recent evidence also supports a role for Keap1 in regulation of Nrf2
degradation (Nguyen et al., 2005). A working model based on current knowledge of cellular signaling
as it relates to the ARE is presented in Figure 6.4.
To our knowledge, no piscine genes have yet been shown to have functional AREs in their regulatory
regions. ARE-like sequences have been identified upstream of the GSTA gene in plaice (Pleuronectes