Page 313 - The Toxicology of Fishes
P. 313
Reactive Oxygen Species and Oxidative Stress 293
(Lipid, containing
R 1 R 2
polyunsaturated
H H fatty acids)
·OH H· abstraction
H O (initiation)
2
R · R
1 2
(Lipid radical)
H H
Diene conjugation
R 1
C·
H
H O 2 addition
R 2
O 2
OO· (Lipid peroxylradical)
R
1 C
H
H R 2
Reaction with second lipid molecule
(propagation)
OOH
R
1 C (Lipid hydroperoxide)
R 2 H
H
+ Second lipid radical
Continued propagation
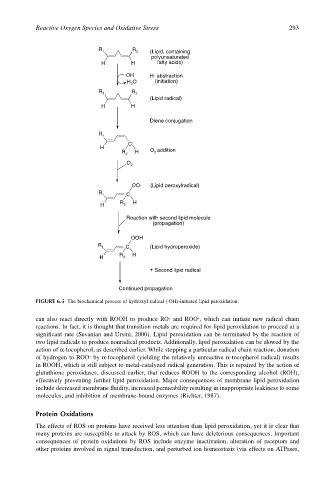
FIGURE 6.5 The biochemical process of hydroxyl radical (·OH)-initiated lipid peroxidation.
can also react directly with ROOH to produce RO· and ROO·, which can initiate new radical chain
reactions. In fact, it is thought that transition metals are required for lipid peroxidation to proceed at a
significant rate (Sevanian and Ursini, 2000). Lipid peroxidation can be terminated by the reaction of
two lipid radicals to produce nonradical products. Additionally, lipid peroxidation can be slowed by the
action of α-tocopherol, as described earlier. While stopping a particular radical chain reaction, donation
of hydrogen to ROO· by α-tocopherol (yielding the relatively unreactive α-tocopherol radical) results
in ROOH, which is still subject to metal-catalyzed radical generation. This is repaired by the action of
glutathione peroxidases, discussed earlier, that reduces ROOH to the corresponding alcohol (ROH),
effectively preventing further lipid peroxidation. Major consequences of membrane lipid peroxidation
include decreased membrane fluidity, increased permeability resulting in inappropriate leakiness to some
molecules, and inhibition of membrane-bound enzymes (Richter, 1987).
Protein Oxidations
The effects of ROS on proteins have received less attention than lipid peroxidation, yet it is clear that
many proteins are susceptible to attack by ROS, which can have deleterious consequences. Important
consequences of protein oxidations by ROS include enzyme inactivation, alteration of receptors and
other proteins involved in signal transduction, and perturbed ion homeostasis (via effects on ATPases,