Page 3 - Demo
P. 3
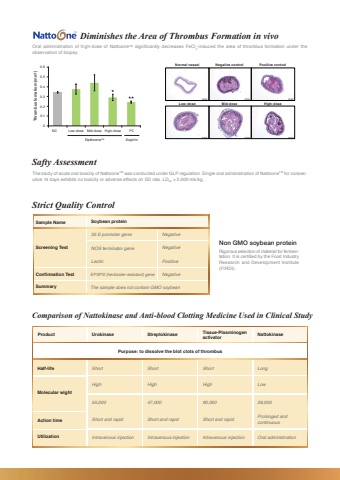
Non GMO soybean proteinSample Name Soybean proteinOral administration of high-dose of NattooneTM significantly decreases FeCl3-induced the area of thrombus formation under the observation of biopsy.Diminishes the Area of Thrombus Formation in vivoThe study of acute oral toxicity of NattooneTM was conducted under GLP regulation. Single oral administration of NattooneTM for consecutive 14 days exhibits no toxicity or adverse effects on SD rats. LD50 > 2,000 mk/kg.Safty AssessmentRigorous selection of material for fermentation. It is certified by the Food Industry Research and Development Institute (FIRDI).Strict Quality Control 00.10.20.30.40.50.6NC Low-dose Mid-dose High-dose PCThrombus fomatiom (mm2)NattooneTM AspirinNormal vessel Negative control Positive controlLow-dose Mid-dose High-doseScreening TestConfirmation TestSummary35 S promoter gene NegativeNegativePositiveNegativeNOS terminator geneLectinEPSPS (herbicide-resistant) geneThe sample does not contain GMO soybeanProduct Urokinase Streptokinase Nattokinase Tissue-Plasminogen activatorPurpose: to dissolve the blot clots of thrombusComparison of Nattokinase and Anti-blood Clotting Medicine Used in Clinical StudyHalf-life ShortHigh55,000Short and rapidIntravenous injectionShortHigh47,000Short and rapidIntravenous injectionShortHigh90,000Short and rapidIntravenous injectionLongLow28,000Prolonged and continuousOral administrationMolecular wightAction timeUtilization