Page 370 - Libro 2
P. 370
350
PART 5 — ABDOMINAL
relatively nontoxic immunosuppressant agent, was introduced and resulted in a significant improvement in patient outcomes by reducing the risk of rejection. Since then, continued evolution and refinement of immunosuppression protocols have even further de- creased the rate of graft loss due to rejection in renal transplant recipients.
Currently, renal transplantation is considered the treatment of choice for the majority of patients with end-stage renal disease (ESRD), providing bet- ter quality of life and long-term survival when com- pared to either peritoneal dialysis or hemodialysis.3–5 Common causes of ESRD include diabetes mellitus, autosomal dominant polycystic kidney disease, glo- merulonephritis, hypertension, atherosclerosis, and systemic lupus erythematosus, with diabetes being the most common cause of kidney transplantation.
According to the Organ Procurement and Trans- plantation Network (OPTN) (http://optn.transplant. hrsa.gov), 16,829 kidney transplants (including both cadaveric and living-related donors) were performed in the United States in 2009. However, there were more than 86,000 people on the waiting list for a kid- ney transplant in 2010. In fact, it is estimated that a new name is added to the waiting list approximately every 13 seconds.4 Thus, organ shortage is the major rate-limiting factor for patients awaiting renal trans- plantation. The current organ shortage has resulted in a loosening of the criteria for deceased donors (DDs), an increase in the use of living-related donors (LRDs), as well as other creative means of increasing organ availability.
There has been a steady increase in graft survival since the first kidney was transplanted in the 1950s due to significant advances in immunosuppression protocols, surgical techniques, and the improve- ment in rapid and efficient organ distribution of HLA-matched DD grafts by the United Network for Organ Sharing (UNOS). In 2010, the OPTN reported a 79.7% 5-year graft survival rate for living-related HLA-matched donors and a 66.5% 5-year graft sur- vival rate for DD grafts.4 Risk factors for graft loss in- clude the number of HLA mismatches, the increased age of donors or recipients, African American race, cold ischemic time greater than 24 hours, and diabet- ic nephropathy as the cause of the recipient’s renal failure.6
Once a person has received a transplant, they are closely monitored for any signs of graft failure or complication. Patients with graft failure most com- monly present with anuria or a rising serum creati- nine level. Pain, tenderness, fever, chills, or elevated white blood cell (WBC) count may also indicate graft dysfunction. However, these are all quite nonspecific signs and symptoms. Hence, imaging, particularly Doppler ultrasound, plays a vital role in the clinical
assessment of graft dysfunction following renal trans- plantation by helping to differentiate anatomic and/ or vascular problems that may require surgical inter- vention from functional abnormalities such as acute tubular necrosis (ATN), drug toxicity, and rejection, which are all treated medically.
THE OPERATION
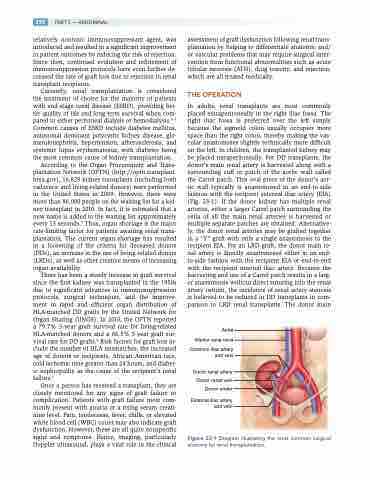
In adults, renal transplants are most commonly placed extraperitoneally in the right iliac fossa. The right iliac fossa is preferred over the left simply because the sigmoid colon usually occupies more space than the right colon, thereby making the vas- cular anastomoses slightly technically more difficult on the left. In children, the transplanted kidney may be placed intraperitoneally. For DD transplants, the donor’s main renal artery is harvested along with a surrounding cuff or patch of the aortic wall called the Carrel patch. This oval piece of the donor’s aor- tic wall typically is anastomosed in an end-to-side fashion with the recipient external iliac artery (EIA) (Fig. 23-1). If the donor kidney has multiple renal arteries, either a larger Carrel patch surrounding the ostia of all the main renal arteries is harvested or multiple separate patches are obtained. Alternative- ly, the donor renal arteries may be grafted together in a “Y” graft with only a single anastomosis to the recipient EIA. For an LRD graft, the donor main re- nal artery is directly anastomosed either in an end- to-side fashion with the recipient EIA or end-to-end with the recipient internal iliac artery. Because the harvesting and use of a Carrel patch results in a larg- er anastomosis without direct suturing into the renal artery ostium, the incidence of renal artery stenosis is believed to be reduced in DD transplants in com- parison to LRD renal transplants. The donor main
Aorta Inferior vena cava
Common iliac artery and vein
Donor renal artery Donor renal vein Donor ureter
External iliac artery and vein
Figure 23-1 Diagram illustrating the most common surgical anatomy for renal transplantation.