Page 130 - Basic _ Clinical Pharmacology ( PDFDrive )
P. 130
116 SECTION II Autonomic Drugs
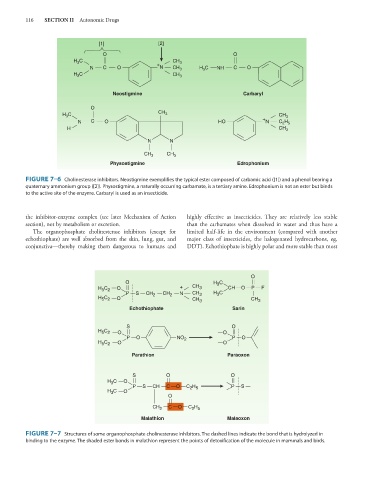
[1] [2]
O O
H C CH 3
3
N C O + N CH 3 H 3 C NH C O
H C CH 3
3
Neostigmine Carbaryl
O
C CH 3
H 3 + CH 3
N C O HO N C H
2 5
H CH 3
N N
CH 3 CH 3
Physostigmine Edrophonium
FIGURE 7–6 Cholinesterase inhibitors. Neostigmine exemplifies the typical ester composed of carbamic acid ([1]) and a phenol bearing a
quaternary ammonium group ([2]). Physostigmine, a naturally occurring carbamate, is a tertiary amine. Edrophonium is not an ester but binds
to the active site of the enzyme. Carbaryl is used as an insecticide.
the inhibitor-enzyme complex (see later Mechanism of Action highly effective as insecticides. They are relatively less stable
section), not by metabolism or excretion. than the carbamates when dissolved in water and thus have a
The organophosphate cholinesterase inhibitors (except for limited half-life in the environment (compared with another
echothiophate) are well absorbed from the skin, lung, gut, and major class of insecticides, the halogenated hydrocarbons, eg,
conjunctiva—thereby making them dangerous to humans and DDT). Echothiophate is highly polar and more stable than most
O
O H C
3
H C O + CH 3 CH O P F
5 2
P S CH 2 CH 2 N CH 3 H C
3
H C O CH 3 CH 3
5 2
Echothiophate Sarin
S O
H C O O
5 2
P O NO 2 P O
H C O O
5 2
Parathion Paraoxon
S O O
H C O
3
P S CH C O C H P S
2 5
H C O
3
O
CH 2 C O C H
2 5
Malathion Malaoxon
FIGURE 7–7 Structures of some organophosphate cholinesterase inhibitors. The dashed lines indicate the bond that is hydrolyzed in
binding to the enzyme. The shaded ester bonds in malathion represent the points of detoxification of the molecule in mammals and birds.