Page 155 - Enzymes in Tropical Soils
P. 155
Enzymes in Tropical Soils 143
+
Na, or NH 4 generally increase Zn release through cation exchange, and hence, may
mobilize Zn in the soil system.
Another possibility is that the increase in soil pH may increase the heavy
metal precipitation because this process may increase with the increase in soil pH
(Lindsay, 1979; Brummer et al., 1983; Ma and Lindsay, 1990; 1995; Workman and
Lindsay, 1990; El-Falaky et al., 1991; Stahl and James, 1991; Salam, 2000).
However, the Zn precipitation forming hidroxydes do not occur at natural
2+
2+
concentration of Zn (Udo et al., 1970). If Zn concentration increases above the
- 2
2+
maximum adsorption capacity, the product of the concentration of (Zn )(OH ) is
well correlated with the solubility product of Zn(OH) 2 , which indicates that Zn
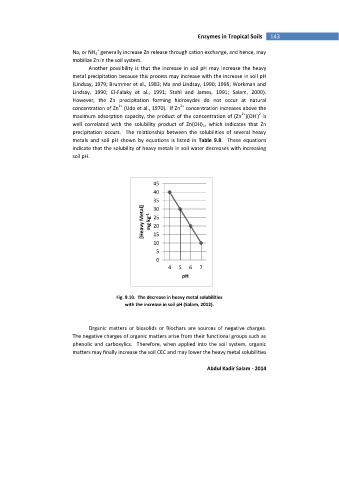
precipitation occurs. The relationship between the solubilities of several heavy
metals and soil pH shown by equations is listed in Table 9.8. These equations
indicate that the solubility of heavy metals in soil water decreases with increasing
soil pH.
45
40
35
[Heavy Metal] mg kg -1 25
30
20
15
10
5
0
4 5 6 7
pH
Fig. 9.10. The decrease in heavy metal solubilities
with the increase in soil pH (Salam, 2012).
Organic matters or biosolids or Biochars are sources of negative charges.
The negative charges of organic matters arise from their functional groups such as
phenolic and carboxylics. Therefore, when applied into the soil system, organic
matters may finally increase the soil CEC and may lower the heavy metal solubilities
Abdul Kadir Salam - 2014