Page 135 - Ilmu Tanah
P. 135
122 The Chemistry and Fertility of Soils under Tropical Weeds
+
interlayers. Potassium ions are then released into the soil solution while H ions
enter the tetrahedral and octahedral positions displacing the cation positions. This
acid dissolution is the main mechanism that weathers the silicate minerals. In an
+
acid condition, 10 H ions may weather 1 molecule of biotite producing 1.5
+
2+
2+
molecules of kaolinite and releasing various cations like K , Fe , and Mg . This
reaction is mathematically expressed in Eq. 7.2 (Stumm and Morgan, 1981).
+
K 2 (Al 2 Si 6 ) (Fe 3 Mg 3 )O 20 (OH) 4 + 4 Al(OH) 2 (H 2 O) 4 + 10 H
2+
2+
+
1.5 Al 4 Si 4 O 10 (OH) 8 + 2 K + 3 Fe + 3 Mg + 2 H 2 O ...... Eq. 7.2
120
100
Release Nutrient (mg kg -1 ) 80
60
40
20
0
2 4 6 8
Soil pH
Soil A Soil B Soil C
Tanah C
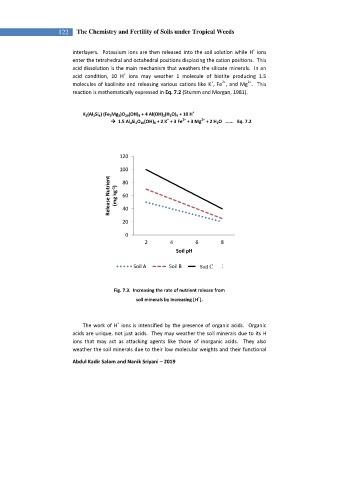
Fig. 7.3. Increasing the rate of nutrient release from
+
soil minerals by increasing [H ].
+
The work of H ions is intensified by the presence of organic acids. Organic
acids are unique, not just acids. They may weather the soil minerals due to its H
ions that may act as attacking agents like those of inorganic acids. They also
weather the soil minerals due to their low molecular weights and their functional
Abdul Kadir Salam and Nanik Sriyani – 2019