Page 133 - Ilmu Tanah
P. 133
120 The Chemistry and Fertility of Soils under Tropical Weeds
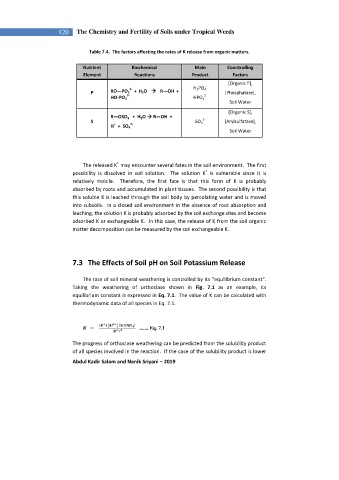
Table 7.4. The factors affecting the rates of K release from organic matters.
Nutrient Biochemical Main Conntrolling
Element Reactions Product Factors
[Organic P],
-
2-
RO—PO 3 + H 2 O R—OH + H 2 PO 4
P 2- 2- [Phosphatase],
HO-PO 3 HPO 4
Soil Water
[Organic S],
-
R—OSO 3 + H 2 O R—OH +
2-
S + 2- SO 4 [Arylsulfatase],
H + SO 4
Soil Water
+
The released K may encounter several fates in the soil environment. The first
+
possibility is dissolved in soil solution. The solution K is vulnerable since it is
relatively mobile. Therefore, the first fate is that this form of K is probably
absorbed by roots and accumulated in plant tissues. The second possibility is that
this soluble K is leached through the soil body by percolating water and is moved
into subsoils. In a closed soil environment in the absence of root absorption and
leaching, the solution K is probably adsorbed by the soil exchange sites and become
adsorbed K or exchangeable K. In this case, the release of K from the soil organic
matter decomposition can be measured by the soil exchangeable K.
7.3 The Effects of Soil pH on Soil Potassium Release
The rate of soil mineral weathering is controlled by its “equilibrium constant”.
Taking the weathering of orthoclase shown in Fig. 7.1 as an example, its
equilibrium constant is expressed in Eq. 7.1. The value of K can be calculated with
thermodynamic data of all species in Eq. 7.1.
+
[ ] [ + ] ⌈ ( ) ⌉
= ....... Eq. 7.1
+
[ ]
The progress of orthoclase weathering can be predicted from the solubility product
of all species involved in the reaction. If the case of the solubility product is lower
Abdul Kadir Salam and Nanik Sriyani – 2019