Page 40 - Ilmu Tanah
P. 40
The Chemistry and Fertility of Soils under Tropical Weeds 27
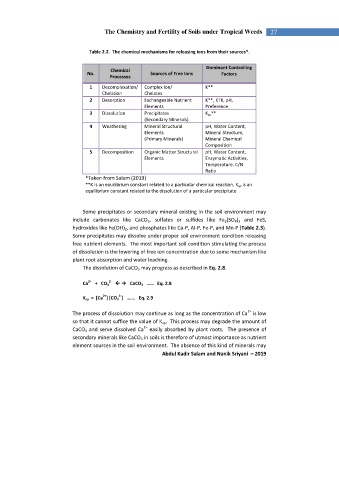
Table 2.2. The chemical mechanisms for releasing ions from their sources*.
Dominant Controlling
Chemical
No. Sources of Free Ions Factors
Processes
1 Decomplexation/ Complex Ion/ K**
Chelation Chelates
2 Desorption Exchangeable Nutrient K**, KTK, pH,
Elements Preference
3 Dissolution Precipitates K sp **
(Secondary Minerals)
4 Weathering Mineral Structural pH, Water Content,
Elements Mineral Structure,
(Primary Minerals) Mineral Chemical
Composition
5 Decomposition Organic Matter Structural pH, Water Content,
Elements Enzymatic Activities,
Temperature, C/N
Ratio
*Taken from Salam (2019)
**K is an equilibrium constant related to a particular chemical reaction, K sp is an
equilibrium constant related to the dissolution of a particular precipitate
Some precipitates or secondary mineral existing in the soil environment may
include carbonates like CaCO 3 , sulfates or sulfides like Fe 2 (SO 4 ) 3 and FeS,
hydroxides like Fe(OH) 2 , and phosphates like Ca-P, Al-P, Fe-P, and Mn-P (Table 2.3).
Some precipitates may dissolve under proper soil environment condition releasing
free nutrient elements. The most important soil condition stimulating the process
of dissolution is the lowering of free ion concentration due to some mechanism like
plant root absorption and water leaching.
The dissolution of CaCO 3 may progress as described in Eq. 2.8.
2-
2+
Ca + CO 3 CaCO 3 …… Eq. 2.8
2-
2+
K sp = [Ca ] [CO 3 ] ……. Eq. 2.9
2+
The process of dissolution may continue as long as the concentration of Ca is low
so that it cannot suffice the value of K sp . This process may degrade the amount of
2+
CaCO 3 and serve dissolved Ca easily absorbed by plant roots. The presence of
secondary minerals like CaCO 3 in soils is therefore of utmost importance as nutrient
element sources in the soil environment. The absence of this kind of minerals may
Abdul Kadir Salam and Nanik Sriyani – 2019