Page 43 - Ilmu Tanah
P. 43
30 The Chemistry and Fertility of Soils under Tropical Weeds
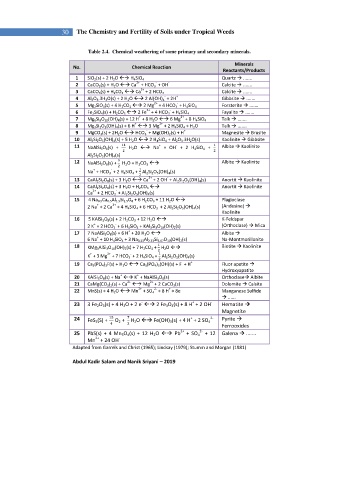
Table 2.4. Chemical weathering of some primary and secondary minerals.
Minerals
No. Chemical Reaction
Reactants/Products
1 SiO 2 (s) + 2 H 2 O H 4 SiO 4 Quartz .......
2+ - -
2 CaCO 3 (s) + H 2 O Ca + HCO 3 + OH Calcite .......
2+ -
3 CaCO 3 (s) + H 2 CO 3 Ca + 2 HCO 3 Calcite .......
- +
4 Al 2 O 3 .3H 2 O(s) + 2 H 2 O 2 Al(OH) 4 + 2H Gibbsite .......
2+ -
5 Mg 2 SiO 4 (s) + 4 H 2 CO 3 2 Mg + 4 HCO 3 + H 4 SiO 4 Forsterite .......
2+ -
6 Fe 2 SiO 4 (s) + H 2 CO 3 2 Fe + 4 HCO 3 + H 4 SiO 4 Fayalite .......
+ 2+
7 Mg 6 Si 8 O 20 (OH) 4 (s) + 12 H + 8 H 2 O 6 Mg + 8 H 4 SiO 4 Talk .......
+ 2+
8 Mg 3 Si 2 O 5 (OH) 4 (s) + 6 H 3 Mg + 2 H 4 SiO 4 + H 2 O Talk .......
- +
9 MgCO 3 (s) + 2H 2 O HCO 3 + Mg(OH) 2 (s) + H Magnesite Brusite
10 Al 2 Si 2 O 5 (OH) 4 (s) + 5 H 2 O 2 H 4 SiO 4 + Al 2 O 3 .3H 2 O(s) Kaolinite Gibbsite
1
-
+
11 NaAlSi 3 O 8 (s) + 11 H 2 O Na + OH + 2 H 4 SiO 4 + Albite Kaolinite
2 2
Al 2 Si 2 O 5 (OH) 4 (s)
9
12 NaAlSi 3 O 8 (s) + H 2 O + H 2 CO 3 Albite Kaolinite
2
1
+
-
Na + HCO 3 + 2 H 4 SiO 4 + Al 2 Si 2 O 5 (OH) 4 (s)
2
2+
-
13 CaAl 2 Si 2 O 8 (s) + 3 H 2 O Ca + 2 OH + Al 2 Si 2 O 5 (OH) 4 (s) Anortit Kaolinite
14 CaAl 2 Si 2 O 8 (s) + 3 H 2 O + H 2 CO 3 Anortit Kaolinite
-
2+
Ca + 2 HCO 3 + Al 2 Si 2 O 5 (OH) 4 (s)
15 4 Na 0.5 Ca 0.5 Al 1.5 Si 2.5 O 8 + 6 H 2 CO 3 + 11 H 2 O Plagioclase
-
2+
+
2 Na + 2 Ca + 4 H 4 SiO 4 + 6 HCO 3 + 2 Al 2 Si 2 O 5 (OH) 4 (s) (Andesine)
Kaolinite
16 5 KAlSi 3 O 8 (s) + 2 H 2 CO 3 + 12 H 2 O K-Feldspar
+ -
2 K + 2 HCO 3 + 6 H 4 SiO 4 + KAl 3 Si 3 O 10 (OH) 2 (s) (Orthoclase) Mica
+
17 7 NaAlSi 3 O 8 (s) + 6 H + 20 H 2 O Albite
+
6 Na + 10 H 4 SiO 4 + 3 Na 0.33 Al 2.33 Si 3.67 O 10 (OH) 2 (s) Na-Montmorillonite
1
18 KMg 3 AlSi 3 O 10 (OH) 2 (s) + 7 H 2 CO 3 + H 2 O Biotite Kaolinite
2 1
-
2+
+
K + 3 Mg + 7 HCO 3 + 2 H 4 SiO 4 + Al 2 Si 2 O 5 (OH) 4 (s)
2
+
-
19 Ca 5 (PO 4 ) 3 F(s) + H 2 O Ca 5 (PO 4 ) 3 (OH)(s) + F + H Fluor apatite
Hydroxyapatite
+
+
20 KAlSi 3 O 8 (s) + Na K + NaAlSi 3 O 8 (s) Orthoclase Albite
2+
2+
21 CaMg(CO 3 ) 2 (s) + Ca Mg + 2 CaCO 3 (s) Dolomite Calsite
2+
+
2-
22 MnS(s) + 4 H 2 O Mn + SO 4 + 8 H + 8e - Manganese Sulfide
......
-
+
23 3 Fe 2 O 3 (s) + 4 H 2 O + 2 e 2 Fe 3 O 4 (s) + 8 H + 2 OH - Hematite
Magnetite
15
7
24 FeS 2 (S) + O 2 + H 2 O Fe(OH) 3 (s) + 4 H + 2 SO 4 Pyrite
+
2-
4 2
Ferrooxides
2-
2+
25 PbS(s) + 4 Mn 3 O 4 (s) + 12 H 2 O Pb + SO 4 + 12 Galena .......
2+
Mn + 24 OH -
Adapted from Garrels and Christ (1965); Lindsay (1979); Stumm and Morgan (1981)
Abdul Kadir Salam and Nanik Sriyani – 2019