Page 154 - Ilmu Tanah Book
P. 154
The Chemistry and Fertility of Soils under Tropical Weeds 141
processes in controlling the concentrations of heavy metals in soil water is
debatable, but are in general depending on the heavy metal cation concentration
and soil pH.
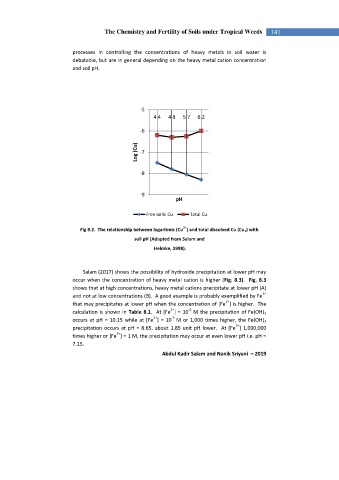
-5
4.4 4.8 5.7 6.2
-6
Log (Cu) -7
-8
-9
pH
Free ionic Cu Total Cu
2+
Fig 8.2. The relationship between logaritmic (Cu ) and total dissolved Cu (Cu T ) with
soil pH (Adapted from Salam and
Helmke, 1998).
Salam (2017) shows the possibility of hydroxide precipitation at lower pH may
occur when the concentration of heavy metal cation is higher (Fig. 8.3). Fig. 8.3
shows that at high concentrations, heavy metal cations precipitate at lower pH (A)
2+
and not at low concentrations (B). A good example is probably exemplified by Fe
2+
that may precipitates at lower pH when the concentration of [Fe ] is higher. The
2+ -6
calculation is shown in Table 8.1. At [Fe ] = 10 M the precipitation of Fe(OH) 2
2+ -3
occurs at pH = 10.15 while at [Fe ] = 10 M or 1,000 times higher, the Fe(OH) 2
3+
precipitation occurs at pH = 8.65, about 1.85 unit pH lower. At [Fe ] 1,000,000
3+
times higher or [Fe ] = 1 M, the precipitation may occur at even lower pH i.e. pH =
7.15.
Abdul Kadir Salam and Nanik Sriyani – 2019