Page 39 - Instrumen Soal HOTS
P. 39
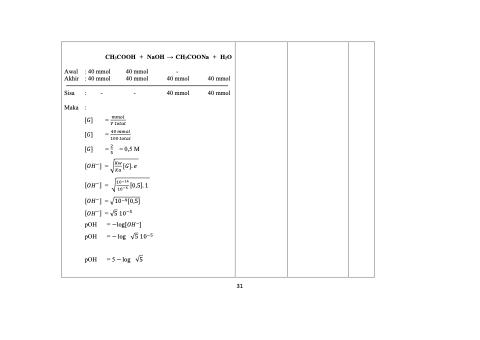
Awal : 40 mmol Akhir : 40 mmol
40 mmol 40 mmol
-
40 mmol
40 mmol
40 mmol 40 mmol
Sisa : Maka :
[𝐺] [𝐺] [𝐺]
-
-
CH3COOH + NaOH → CH3COONa + H2O
= 𝑚𝑚𝑜𝑙 𝑉 𝑡𝑜𝑡𝑎𝑙
= 40 𝑚𝑚𝑜𝑙 100 𝑡𝑜𝑡𝑎𝑙
= 2 = 0,5 M 5
[𝑂𝐻−] =
√𝐾𝑤 [𝐺]. 𝑒 𝐾𝑎
[𝑂𝐻−] =
√10−14 [0,5]. 1 10−5
[𝑂𝐻−] =
√10−9 [0,5]
[𝑂𝐻−] = √5 10−5
pOH = − log √5 10−5 pOH =5−log √5
pOH =
−log[𝑂𝐻−]
31