Page 46 - Environment: The Science Behind the Stories
P. 46
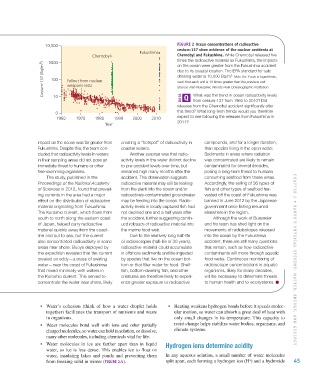
10,000 Figure 2 ocean concentrations of radioactive
cesium-137 show evidence of the nuclear accidents at
Fukushima
Chernobyl chernobyl and Fukushima. While Chernobyl released five
times the radioactive material as Fukushima, the impacts
1000
Cesium-137 (Bq/m 3 ) 100 Fallout from nuclear due to its coastal location. The EPA standard for safe
on the ocean were greater from the Fukushima accident
3.
drinking water is 10,000 Bq/m Note the Y axis is logarithmic,
such that each unit is 10 times greater than the previous unit.
weapons tests
Source: Ken Buesseler, Woods Hole Oceanographic Institution.
What was the trend in ocean radioactivity levels
10
from cesium-137 from 1960 to 2010? Did
releases from the Chernobyl accident significantly alter
0 this trend? What long-term trends would you therefore
1960 1970 1980 1990 2000 2010 expect to see following the releases from Fukushima in
Year 2011?
impact on the ocean was far greater from creating a “hotspot” of radioactivity in compounds, and for a longer duration,
Fukushima. Despite this, the team con- coastal waters. than species living in the open water.
cluded that radioactivity levels in waters Another surprise was that radio- Sediments in areas where radiation
in their sampling areas did not pose an activity levels in the water did not decline was concentrated are likely to remain
immediate threat to humans or other to pre-accident levels over time, but contaminated for several decades,
free-swimming organisms. remained high many months after the posing a long-term threat to humans
The study, published in the accident. This observation suggests consuming seafood from these areas.
Proceedings of the National Academy radioactive material may still be leaking Accordingly, the selling of 36 types of
of Sciences in 2012, found that prevail- from the plant into the ocean and/or fish and other types of seafood har-
ing currents in the area had a major radioactively-contaminated groundwater vested off the coast of Fukushima was
effect on the distribution of radioactive may be feeding into the ocean. Radio- banned in June 2012 by the Japanese
material originating from Fukushima. activity levels in locally captured fish had government once fishing resumed
The Kuroshio current, which flows from not declined one and a half years after elsewhere in the region. CHAPTER 2 • E ART h’s Physi CAL
south to north along the eastern coast the accident, further suggesting contin- Although the work of Buesseler
of Japan, helped carry radioactive ued releases of radioactive material into and his team has shed light on the
material quickly away from the coast- the marine food web. movements of radioisotopes released
line and out to sea, but the current Due to the relatively long half-life into the ocean by the Fukushima
also concentrated radioactivity in some of radioisotopes (half-life of 30 years), accident, there are still many questions
areas near shore. Buoys deployed by radioactive material could accumulate that remain, such as how radioactive
the expedition revealed that the current in offshore sediments and be ingested contaminants will move through aquatic
created an eddy—a mass of swirling by species that live on the ocean bot- food webs. Continuous monitoring of
water—near the coast of Fukushima tom or that filter water for food. Shell- radioisotope concentrations in aquatic
that mixed minimally with waters in fish, bottom-dwelling fish, and other organisms, likely for many decades,
the Kuroshio current. This served to creatures are therefore likely to experi- will be necessary to determine threats
concentrate the water near shore, likely ence greater exposure to radioactive to human health and to ecosystems. s ys TE m s: mATTER , E NER gy, AN d
• Water’s cohesion (think of how a water droplet holds • Heating weakens hydrogen bonds before it speeds molec-
together) facilitates the transport of nutrients and waste ular motion, so water can absorb a great deal of heat with
in organisms. only small changes in its temperature. This capacity to
• Water molecules bond well with ions and other partially resist change helps stabilize water bodies, organisms, and
charged molecules, so water can hold in solution, or dissolve, climate systems.
many other molecules, including chemicals vital for life. gE o L ogy
• Water molecules in ice are farther apart than in liquid Hydrogen ions determine acidity
water, so ice is less dense. This enables ice to float on
water, insulating lakes and ponds and preventing them In any aqueous solution, a small number of water molecules
+
from freezing solid in winter (Figure 2.5). split apart, each forming a hydrogen ion (H ) and a hydroxide 45
M02_WITH7428_05_SE_C02.indd 45 12/12/14 2:53 PM