Page 575 - Environment: The Science Behind the Stories
P. 575
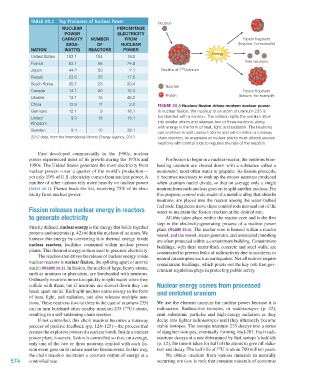
TABLE 20.1 Top Producers of Nuclear Power Neutron
NUCLEAR PERCENTAGE
POWER ELECTRICITY
CAPACITY NUMBER FROM Fission fragment
(GIGA- OF NUCLEAR (krypton, for example)
NATION WATTS) REACTORS POWER
Energy
United States 102.1 104 19.0
France 63.1 58 74.8 Free neutrons
Japan 44.2 50 2.1 Nucleus of 235 Uranium
Russia 23.6 33 17.8
South Korea 20.7 23 30.4 Neutron
Canada 14.1 20 15.3 Fission fragment
Ukraine 13.1 15 46.2 Proton (barium, for example)
China 12.9 17 2.0 FIGURE 20.3 Nuclear fission drives modern nuclear power.
Germany 12.1 9 16.1 In nuclear fission, the nucleus of an atom of uranium-235 is
United 9.9 18 18.1 bombarded with a neutron. The collision splits the uranium atom
Kingdom into smaller atoms and releases two or three neutrons, along
with energy in the form of heat, light, and radiation. The neutrons
Sweden 9.4 10 38.1
can continue to split uranium atoms and set in motion a runaway
2012 data, from the International Atomic Energy Agency, 2013. chain reaction, so engineers at nuclear plants must absorb excess
neutrons with control rods to regulate the rate of the reaction.
First developed commercially in the 1950s, nuclear
power experienced most of its growth during the 1970s and For fission to begin in a nuclear reactor, the neutrons bom-
1980s. The United States generates the most electricity from barding uranium are slowed down with a substance called a
nuclear power—over a quarter of the world’s production— moderator, most often water or graphite. As fission proceeds,
yet only 19% of U.S. electricity comes from nuclear power. A it becomes necessary to soak up the excess neutrons produced
number of other nations rely more heavily on nuclear power when uranium nuclei divide, so that on average only a single
(TABLE 20.1). France leads the list, receiving 75% of its elec- neutron from each nucleus goes on to split another nucleus. For
tricity from nuclear power. this purpose, control rods, made of a metallic alloy that absorbs
neutrons, are placed into the reactor among the water-bathed
Fission releases nuclear energy in reactors fuel rods. Engineers move these control rods into and out of the
water to maintain the fission reaction at the desired rate.
to generate electricity All this takes place within the reactor core and is the first
step in the electricity-generating process of a nuclear power
Strictly defined, nuclear energy is the energy that holds together plant (FIGURE 20.4). The reactor core is housed within a reactor
protons and neutrons (p. 42) within the nucleus of an atom. We vessel, and the vessel, steam generator, and associated plumbing
harness this energy by converting it to thermal energy inside are often protected within a containment building. Containment
nuclear reactors, facilities contained within nuclear power buildings, with their meter-thick concrete and steel walls, are
plants. This thermal energy is then used to generate electricity. constructed to prevent leaks of radioactivity due to accidents or
The reaction that drives the release of nuclear energy inside natural catastrophes such as earthquakes. Not all nations require
nuclear reactors is nuclear fission, the splitting apart of atomic containment buildings, which points out the key role that gov-
nuclei (FIGURE 20.3). In fission, the nuclei of large, heavy atoms, ernment regulation plays in protecting public safety.
such as uranium or plutonium, are bombarded with neutrons.
Ordinarily neutrons move too quickly to split nuclei when they
collide with them, but if neutrons are slowed down they can Nuclear energy comes from processed
break apart nuclei. Each split nucleus emits energy in the form and enriched uranium
of heat, light, and radiation, and also releases multiple neu-
trons. These neutrons (two to three in the case of uranium-235) We use the element uranium for nuclear power because it is
can in turn bombard other nearby uranium-235 ( U) atoms, radioactive. Radioactive isotopes, or radioisotopes (p. 42),
235
resulting in a self-sustaining chain reaction. emit subatomic particles and high-energy radiation as they
If not controlled, this chain reaction becomes a runaway decay into lighter radioisotopes until they ultimately become
process of positive feedback (pp. 124–125)—the process that stable isotopes. The isotope uranium-235 decays into a series
creates the explosive power of a nuclear bomb. Inside a nuclear of daughter isotopes, eventually forming lead-207. Each radi-
power plant, however, fission is controlled so that, on average, oisotope decays at a rate determined by that isotope’s half-life
only one of the two or three neutrons emitted with each fis- (p. 42), the time it takes for half of the atoms to give off radia-
235
sion event goes on to induce another fission event. In this way, tion and decay. The half-life of U is about 700 million years.
the chain reaction maintains a constant output of energy at a We obtain uranium from various minerals in naturally
574 controlled rate. occurring ore (ore is rock that contains minerals of economic
M20_WITH7428_05_SE_C20.indd 574 13/12/14 1:56 PM