Page 11 - Fuel Cell Student Edition
P. 11
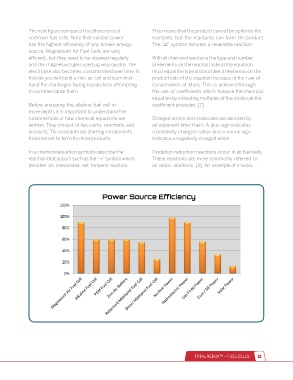
The next figure compares the efficiencies of This means that the product cannot be split into the
common fuel cells. Note that nuclear power reactants, but the reactants can form the product.
has the highest efficiency of any known energy The “ ” symbol denotes a reversible reaction.
source. Magnesium-Air Fuel Cells are very
efficient, but they need to be cleaned regularly With all chemical reactions the type and number
and the magnesium gets used up very quickly. The of elements on the reactant side of the equation
electrolyte also becomes contaminated over time. In must equal the type and number of elements on the
this lab you will build a zinc-air cell and learn first- product side of the equation because of the Law of
hand the challenges facing researchers attempting Conservation of Mass. This is achieved through
to commercialize them. the use of coefficients which balance the chemical
equation by indicating multiples of the molecule the
Before analyzing the alkaline fuel cell in coefficient precedes. [7]
more depth, it is important to understand the
fundamentals of how chemical equations are Charged atoms and molecules are denoted by
written. They consist of two parts: reactants and an exponent after them. A plus sign indicates
products. The reactants are starting components a positively charged cation and a minus sign
that interact to form the final products. indicates a negatively charged anion.
In a chemical equation symbols describe the Oxidation-reduction reactions occur in all fuel cells.
reaction that occurs such as the “ ” symbol which These reactions are more commonly referred to
denotes an irreversible net forward reaction. as redox reactions. [8] An example of a redox
10 TOTAL REDOX™ – FUEL CELLS TOTAL REDOX™ – FUEL CELLS 11