Page 12 - Fuel Cell Student Edition
P. 12
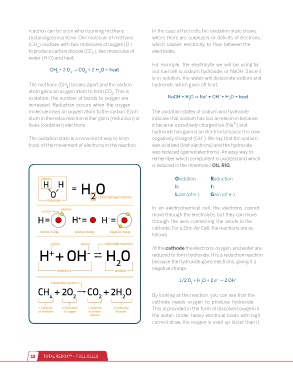
reaction can be seen when burning methane In the case of fuel cells the oxidation state shows
(natural gas) in a stove. One molecule of methane where there are surpluses or deficits of electrons,
(CH ) oxidizes with two molecules of oxygen (O ) which causes electricity to flow between the
4
to produce carbon dioxide (CO ), two molecules of electrodes.
2
water (H O) and heat.
For example, the electrolyte we will be using for
our fuel cell is sodium hydroxide, or NaOH. Since it
is in solution, the water will dissociate sodium and
The methane (CH ) breaks apart and the carbon hydroxide, which gives off heat.
4
atom gains an oxygen atom to form CO . This is
2
oxidation; the number of bonds to oxygen are
increased. Reduction occurs when the oxygen
molecule loses an oxygen atom to the carbon. Each The oxidation states of sodium and hydroxide
atom in the redox reaction either gains (reduction) or indicate that sodium has lost an electron because
+
loses (oxidation) electrons. it became a positively charged ion (Na ) and
hydroxide has gained an electron because it is now
-
The oxidation state is a convenient way to keep negatively charged (OH ). We say that the sodium
track of the movement of electrons in the reaction. was oxidized (lost electrons) and the hydroxide
was reduced (gained electrons). An easy way to
remember which component is oxidized and which
is reduced is the mnemonic OIL RIG.
Oxidation Reduction
Is Is
Loss (of e-) Gain (of e-)
In an electrochemical cell, the electrons cannot
move through the electrolyte, but they can move
through the wire connecting the anode to the
cathode. For a Zinc-Air Cell, the reactions are as
follows:
At the cathode the electrons, oxygen, and water are
reduced to form hydroxide. It is a reduction reaction
because the hydroxide gains electrons, giving it a
negative charge.
By looking at the reaction, you can see that the
cathode needs oxygen to produce hydroxide.
This is provided in the form of dissolved oxygen in
the water. Under heavy electrical loads with high
current draw, the oxygen is used up faster than it
12 TOTAL REDOX™ – FUEL CELLS TOTAL REDOX™ – FUEL CELLS 13