Page 59 - GMP for warehouse
P. 59
GMP Training – GMP for Warehouse by www.gmpsop.com
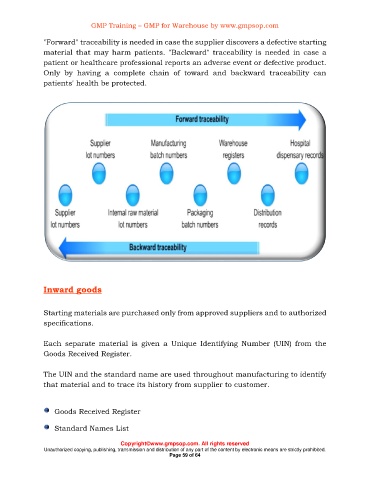
"Forward" traceability is needed in case the supplier discovers a defective starting
material that may harm patients. "Backward" traceability is needed in case a
patient or healthcare professional reports an adverse event or defective product.
Only by having a complete chain of toward and backward traceability can
patients' health be protected.
Inward goods
Starting materials are purchased only from approved suppliers and to authorized
specifications.
Each separate material is given a Unique Identifying Number (UIN) from the
Goods Received Register.
The UIN and the standard name are used throughout manufacturing to identify
that material and to trace its history from supplier to customer.
Goods Received Register
Standard Names List
Copyright©www.gmpsop.com. All rights reserved
Unauthorized copying, publishing, transmission and distribution of any part of the content by electronic means are strictly prohibited.
Page 59 of 64