Page 112 - 00. Complete Version - Progress Report IPEN 2014-2016
P. 112
112 Renewable Energies | Progress Report
Highlights 2014-2016
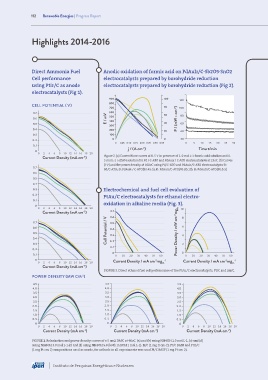
Direct Ammonia Fuel Anodic oxidation of formic acid on PdAuIr/C-Sb2O5·SnO2
Cell performance electrocatalysts prepared by borohydride reduction
using PtIr/C as anode electrocatalysts prepared by borohydride reduction (Fig 2).
electrocatalysts (Fig 1).
900 100 120
CELL POTENTIAL ( V) 800
700 80 100
0.7 600 60
500
0.6 E / mV 400 P / (mW • cm -2 ) 80
0.5 40 60
300
0.4 200 20 40
0.3 100
0. 2 0 0 20
0 0.05 0.10 0.15 0.20 0.25 0.30 0.35 0 5 10 15 20 25 30
0. 1
j / (A cm ) Time t/min
-2
0
0 2 4 6 8 10 12 14 16 18 20
Current Density (mA cm ) Figure 2. (a) Current time curves at 0. 5 V in presence of 1. 0 mol L-1 formic acid solution and 0.
-2
5 mol L-1 H2SO4 solution for Pd / C-ATO and PdAuIr / C-ATO electrocatalysts at 25oC. (b) Curves
(I-V) and the power density at 100oC using Pd/C-ATO and PdAuIr/C-ATO electrocatalysts ▲:
0.7
Pd/C-ATO; ▲: PdAuIr / C-ATO(50:45:5); ▲: PdAuIr/C-ATO(70:20:10); ▲: PdAuIr/C-ATO(90:5:5)
0.6
0.5
0.4
0.3 Electrochemical and fuel cell evaluation of
0. 2
PtAu/C electrocatalysts for ethanol electro-
0. 1
0 oxidation in alkaline media (Fig. 3).
-1
0 2 4 6 8 10 12 14 16 18 20 10
Current Density (mA cm ) 0.7
-2
0.7 0.6 8
0.5
0.6 0.4 6
0.5 Cell Potential / V 0.3 Power Density / mW cm -2 mg Pt 4
0.4 0. 2
0.3 2
0. 1
0. 2
0 0
0. 1 0 10 20 30 40 50 60 0 10 20 30 40 50 60
0 Current Density / mA cm mg -1 Current Density / mA cm mg -1
-2
-2
0 2 4 6 8 10 12 14 16 18 20 Pt Pt
Current Density (mA cm )
-2
FIGURE 3. Direct ethanol fuel cell performance of the PtAu/C electrocatalysts, Pt/C and Au/C.
POWER DENSITY (MW CM )
-2
4.5 4.5 4.5
4.0 4.0 4.0
3.5 3.5 3.5
3.0 3.0 3.0
2.5 2.5 2.5
2. 0 2. 0 2. 0
1. 5 1. 5 1. 5
1. 0 1. 0 1. 0
0. 5 0. 5 0. 5
0 0 0
0 2 4 6 8 10 12 14 16 18 20 0 2 4 6 8 10 12 14 16 18 20 0 2 4 6 8 10 12 14 16 18 20
Current Density (mA cm ) Current Density (mA cm ) Current Density (mA cm )
-2
-2
-2
FIGURE 1. Polarization and power density curves of a 5 cm2 DAFC at 40oC. (a) and (b) using NH4OH 1.0 mol L-1. (c) and (d)
using NH4OH 3.0 mol L-1.(e) and (f) using NH4OH 5.0 (both in KOH 1 mol L-1). Ir/C (1 mg Ir cm-2), Pt/C BASF and PtIr/C
(1 mg Pt cm-2) compositions used as anode, for cathode in all experiments was used Pt/C BASF (1 mg Pt cm-2).
Instituto de Pesquisas Energéticas e Nucleares