Page 96 - 00. Complete Version - Progress Report IPEN 2014-2016
P. 96
96 Biotechnology | Progress Report
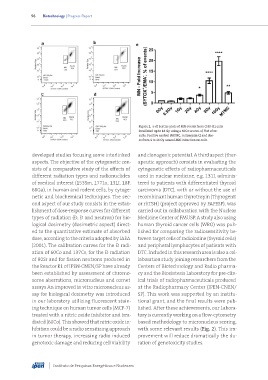
Figure 2. a-d) Scatter plots of MN events from CHO-K1 cells
irradiated up to 16 Gy using a 60Co source. e) Plot of re-
sults. Positive control (MTMC, mitomycin C) and dos-
es from 4 to 16 Gy caused MN induction on cells.
developed studies focusing some interlinked and clonogenic potential. A third aspect (ther-
aspects. The objective of the cytogenetic con- apeutic approach) consists in evaluating the
sists of a comparative study of the effects of cytogenetic effects of radiopharmaceuticals
different radiation types and radionuclides used in nuclear medicine, e.g. 131I, adminis-
of medical interest (153Sm, 177Lu, 131I, 18F, tered to patients with differentiated thyroid
68Ga), in human and rodent cells, by cytoge- carcinoma (DTC), with or without the use of
netic and biochemical techniques. The sec- recombinant human thyrotropin (ThyrogenR
ond aspect of our study consists in the estab- or rhTSH) (project approved by FAPESP), was
lishment of dose-response curves for different carried out in collaboration with the Nuclear
types of radiation (α, α and neutron) for bio- Medicine Center of FMUSP. A study also using
logical dosimetry (dosimetric aspect) direct- human thyroid cancer cells (WRO) was pub-
ed to the quantitative estimate of absorbed lished for comparing the radiosensitivity be-
dose, according to the criteria adopted by IAEA tween target cells of radioiodine (thyroid cells)
(2001). The calibration curves for the α radi- and peripheral lymphocytes of patients with
ation of 60Co and 137Cs, for the α radiation DTC. Included in this research area is also a col-
of 90Sr and for fission neutrons produced in laboration study joining researchers from the
the Reactor R1 of IPEN-CNEN/SP have already Centers of Biotechnology and Radio pharma-
been established by assessment of chromo- cy and the Biosintesis Laboratory for pre-clin-
some aberrations, micronucleus and comet ical trials of radiopharmaceuticals produced
assays An improved in vitro micronucleus as- at the Radiopharmacy Center (IPEN-CNEN/
say for biological dosimetry was introduced SP). This work was supported by an institu-
in our laboratory utilizing fluorescent stain- tional grant, and the final results were pub-
ing technique on human tumor cells (MCF-7) lished. After these achievements, our Labora-
treated with a nitric oxide inhibitor and irra- tory is currently working on a flow-cytometry
diated (60Co). This showed that nitric oxide in- based methodology to micronucleus scoring,
hibition could be a radio sensitizing approach with some relevant results (Fig. 2). This im-
in tumor therapy, increasing radio induced provement will reduce dramatically the du-
genotoxic damage and reducing cell viability ration of genotoxicity studies.
Instituto de Pesquisas Energéticas e Nucleares