Page 424 - SEC_2017WorkingDocument_Neat
P. 424
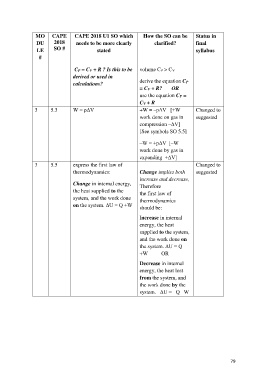
MO CAPE CAPE 2018 U1 SO which How the SO can be Status in
DU 2018 needs to be more clearly clarified? final
LE SO # stated syllabus
#
CP = CV + R ? Is this to be volume CP > CV
derived or used in
calculations? derive the equation CP
= CV + R? OR
use the equation CP =
CV + R
3 5.3 W = pΔV +W = –pΔV [+W Changed to
work done on gas in suggested
compression –ΔV]
[See symbols SO 5.5]
–W = +pΔV [–W
work done by gas in
expanding +ΔV]
3 5.5 express the first law of Changed to
thermodynamics: Change implies both suggested
increase and decrease,
Change in internal energy, Therefore
the heat supplied to the the first law of
system, and the work done thermodynamics
on the system. ΔU = Q +W
should be:
Increase in internal
energy, the heat
supplied to the system,
and the work done on
the system. ΔU = Q
+W OR
Decrease in internal
energy, the heat lost
from the system, and
the work done by the
system. –ΔU = –Q –W
79