Page 464 - SUBSEC October 2017_Neat
P. 464
02212020/CAPE/SPEC/MS/2017
- 6 –
CHEMISTRY
UNIT 2 — PAPER 02
MARK SCHEME
S.O: Module 3: — 1.3, 4.1, 8.4, 8.5, 9.6, 9.7
KC UK XS
(a) (i) Fertilisers, decaying plants or animals 2
(ii) Add aluminum metal or zinc metal followed by sodium
hydroxide solution and warm. If nitrate ions are
present, ammonia gas is produced OR Add copper
turnings followed by conc. Sulphuric acid poured
2
carefully down the side of the testy tube. If nitrate
ions are present a brown ring is produced. [2 marks]
-
(iii) NO2 [1 mark] 1
(iv) Pollutants are usually present in trace (small)
quantities. School laboratory tests are not As
sensitive as the cadmium reduction method to small 2
quantities of NO3 . [2 marks]
-
(b) (i) In urban centres, there is a heavy flow of traffic.
The burning of fuels in the internal combustion engine
is an exothermic reaction, and the high temperatures
produced provide the conditions that favour the
forward endothermic reaction for the production of NO
from nitrogen and oxygen that are present in the air.
2
[2 marks]
(ii) NO is produced first as the primary pollutant after
which NO2 is formed by the oxidation of NO. [1 mark]
1
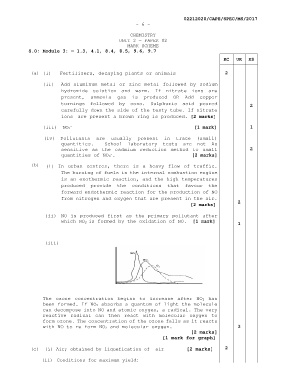
(iii)
The ozone concentration begins to increase after NO2 has
been formed. If NO2 absorbs a quantum of light the molecule
can decompose into NO and atomic oxygen, a radical. The very
reactive radical can then react with molecular oxygen to
form ozone. The concentration of the ozone falls as it reacts
with NO to re-form NO2 and molecular oxygen. 3
[2 marks]
[1 mark for graph]
(c) (i) Air; obtained by liquefication of air [2 marks] 2
(ii) Conditions for maximum yield: