Page 626 - SUBSEC October 2017_Neat
P. 626
- 13 -
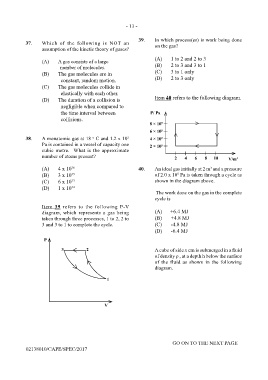
39. In which process(es) is work being done
37. Which of the following is NOT an on the gas?
assumption of the kinetic theory of gases?
(A) 1 to 2 and 2 to 3
(A) A gas consists of a large
number of molecules. (B) 2 to 3 and 3 to 1
(B) The gas molecules are in (C) 3 to 1 only
constant, random motion. (D) 2 to 3 only
(C) The gas molecules collide in
elastically with each other.
(D) The duration of a collision is Item 40 refers to the following diagram.
negligible when compared to
the time interval between
collisions.
38. A monatomic gas at 18 C and 1.2 x 10 5
o
Pa is contained in a vessel of capacity one
cubic metre. What is the approximate
number of atoms present?
(A) 4 x 10 26 40. An ideal gas initially at 2 m and a pressure
3
(B) 3 x 10 25 of 2.0 x 10 Pa is taken through a cycle as
5
(C) 6 x 10 23 shown in the diagram above.
(D) 1 x 10 24
The work done on the gas in the complete
cycle is
Item 39 refers to the following P-V
diagram, which represents a gas being (A) +6.4 MJ
taken through three processes, 1 to 2, 2 to (B) +4.8 MJ
3 and 3 to 1 to complete the cycle. (C) -4.8 MJ
(D) -6.4 MJ
A cube of side x cm is submerged in a fluid
of density ρ , at a depth h below the surface
of the fluid as shown in the following
diagram.
GO ON TO THE NEXT PAGE
02138010/CAPE/SPEC/2017