Page 8 - HSP-Assure Test Booklet FDA Auth Booklet - FINAL 7_2020
P. 8
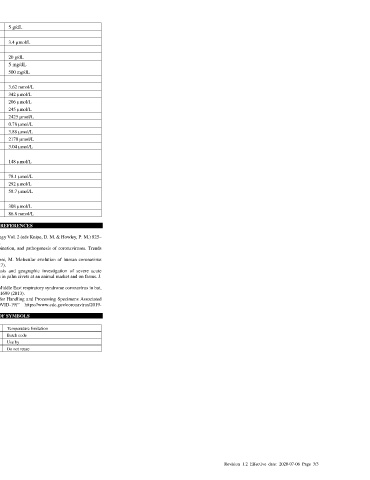
antibody-negative samples without HIV (for this, a confidence interval for the difference in false positive Albumin 5 g/dL
rates was calculated per a score method described by Altman). The results and data analysis are shown in
the Tables 5 and 6 below. Anticoagulants
EDTA (sodium salt) 3.4 µmol/L
Table 5. Summary Results Abnormal blood sample
Assure COVID-19 Comparator Method
IgG/IgM Rapid Test Positive Negative Negative, Total Visual hemolysis (Hemoglobin) 20 g/dL
Device (IgM/IgG) + (IgM/IgG) - HIV+ Icteric (Bilirubin) 5 mg/dL
Positive IgM+/IgG+ 27 0 0 27 Lipemic (Triglycerides) 500 mg/dL
IgM+, IgG- 3 1 0 4 Common medicines
IgM-, IgG+ 0 0 0 0
Negative IgM-/IgG- 0 69 10 79 Acetylsalicylic acid 3.62 mmol/L
Total (n=110) 30 70 10 110 Ascorbic acid (Vitamin C) 342 µmol/L
Amoxicillin 206 µmol/L
Table 6. Summary Statistics Fluconazole 245 µmol/L
Measure Estimate Confidence Interval Ibuprofen 2425 µmol/L
IgM+ Sensitivity (PPA) (30/30) 100% (88.7%; 100%) Loratadine 0.78 µmol/L
IgM- Specificity (NPA) (79/80) 98.8% (93.3%; 98.8%) Nadolol 3.88 µmol/L
IgG+ Sensitivity (PPA) (27/30) 90.0% (74.4%; 96.5%) Naproxen 2170 µmol/L
IgG- Specificity (NPA) (80/80)100% (95.4%; 100%)
Combined Sensitivity (30/30) 100% (88.7%; 100%) Paroxetine 3.04 µmol/L
Combined Specificity (79/80) 98.8% (93.3%; 98.8%) Anti-malarial medicines
Combined PPV for prevalence = 5% 80.8% (40.9%; 96%) Quinine 148 µmol/L
Combined NPV for prevalence = 100% (99.4%; 100%) Anti-tuberculosis medicines
5% Rifampicin 78.1 µmol/L
(0/10) 0%
Cross-reactivity with HIV+ ----------------- Isoniazid 292 µmol/L
not detected Ethambutol 58.7 µmol/L
Cross Reactivity Common consumables
There was no cross-reactivity with plasma specimens meeting the disease state shown below. No IgM or Coffee (caffeine) 308 µmol/L
IgG false positive results were observed with the following potential cross-reactants: Alcohol (ethanol) 86.8 mmol/L
Table 7. Cross-reactivity Study Data of Assure COVID-19 IgG/IgM Rapid Test Device LITERATURE REFERENCES
Number of Number of
Conditions samples Conditions samples 1. Masters, P. S. & Perlman, S. in Fields Virology Vol. 2 (eds Knipe, D. M. & Howley, P. M.) 825–
Anti-HAV IgM + 5 Lyme disease+ 5 858 (Lippincott Williams & Wilkins, 2013).
Anti-HEV IgG + 2 P. falciparum + 5 2. Su, S. et al. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends
Microbiol. 24, 490–502 (2016).
HBsAg + 5 P. vivax + 5 3. Forni, D., Cagliani, R., Clerici, M. & Sironi, M. Molecular evolution of human coronavirus
Anti-HCV + 5 Toxoplasma IgM + 5 4. genomes. Trends Microbiol. 25, 35–48 (2017).
Kan, B. et al. Molecular evolution analysis and geographic investigation of severe acute
Anti-HIV + 5 HAMA + 1 respiratory syndrome coronavirus-like virus in palm civets at an animal market and on farms. J.
Anti-Rubella IgM + 5 RF + 5 Virol. 79, 11892–11900 (2005).
Anti-CMV IgM + 5 ANA+ 5 5. Ithete, N. L. et al. Close relative of human Middle East respiratory syndrome coronavirus in bat,
South Africa. Emerg. Infect. Dis. 19, 1697–1699 (2013).
Anti-HSV-I IgM + 5 Anti-Influenza A IgM + 3 6. “Interim Laboratory Biosafety Guidelines for Handling and Processing Specimens Associated
Anti-HSV-II IgM + 5 Anti-Influenza B IgM + 1 with Coronavirus Disease 2019 (COVID-19)” https://www.cdc.gov/coronavirus/2019-
EBV IgM + 4 Anti-RSV IgM + 3 nCoV/lab/lab-biosafety-guidelines.html.
GLOSSARY OF SYMBOLS
Anti-Dengue IgM + 5 Legionella pneumophila IgM+ 2
Anti-Yellow fever + 5 Anti-Adenovirus IgM + 1 ρ Catalog number 8 Temperature limitation
5 Anti-Mycoplasma pneumonia 3 ι Consult instructions for use Λ Batch code
Anti-Zika IgG +
IgM + Ι In vitro diagnostic medical device ε Use by
5 Anti-Chlamydia pneumonia 3 µ Manufacturer σ Do not reuse
Chagas Ab+
IgM +
4 Anti-Chlamydia pneumonia 2 Distributed by:
Anti-Syphilis IgG + BioLab Sciences
IgG +
Anti-Tuberculosis + 5 Measles IgG + 1 13825 N. Northsight Blvd. #101
Scottsdale, AZ 85260
Typhoid IgM + 5 Mumps IgG + 1 www.biolabsciences.net
4809353744
Interfering Substances
The assay performance of COVID-19 IgG/IgM Rapid Test Device is not affected by substances at
concentrations listed below.
Table 7. Interference Study Data of Assure COVID-19 IgG/IgM Rapid Test
Device@diareagent.com
Interfering substances Concentration of analyate
Blood analytes
Revision 1.2 Effective date: 2020-07-06 Page 3/3